HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Aspirin to...
Hey everyone, awesome forum!
I had an idea similar to the extract benzene thread: Aspirin to "."
Acetylsalicylic acid is a great starting point for many reactions due to its versatility and aspirin is a cheap source of it. I think there should be
a thread sort of like the benzene one. This thread could list experiments that can be done with aspirin as a starting point. the
thread could start with the humble extraction of ASA from aspirin + purification, Then hydrolysis to salicylic acid, to phenol (UC's Prep) etc. Other
experiments could include metal ion complexes (with SA), copper aspirinate, esterification the list goes on..
eventually it could become a publication with many user made experiments. I'm sure many of you have great write ups that use aspirin as a starting
point etc... What do you think? suggestion and experiments are welcome. keen to hear ideas!
note: hopefully this was posted in the right place. please move if not.
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
this is a great thread on the synthesis of copper asprinate from purified acetesalysilic acid, the procedure for the purification of aspirin is also
covered in this thread.
http://www.sciencemadness.org/talk/viewthread.php?tid=9920
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I have a couple folders in my file cabinet dedicated to papers on aspirin. I'll see if I can gather some links, references, and existing
topics. It took me a minute to find the thread you're referring
to. For anyone else who doesn't know: <strong><a href="viewthread.php?tid=4902">Extract of Benzene thread</a></strong>
[edit] I can't access my file cabinet. It could be a while... if I remember at all.
[Edited on 12.12.13 by bfesser]
|
|
|
The_Davster
|
Thread Moved
6-12-2013 at 21:08 |
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Nitrate in mild conditions and then separate the 3-nitro and 5-nitro salicylic acids. Good substrate for column chromatography, as both compounds are
yellow.
Get enough of the nitrosalycilates and you can reduce them to the corresponding aminosalycilates.
You can diazotize the amino compounds and couple them with something like 2-naphthol to make two different azo dyes. Permanently dye something by
painting it with 2-naphthol and then dipping in a solution of the diazonium mix.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You are welcome to review the literature and add the results to the index here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=27...
https://www.sciencemadness.org/whisper/viewthread.php?tid=27...
It is a project that is still (unsuccessfully) trying to get a life, but lacking any volunteer to do the work. It is great that you are interested in
doing it for aspirin (and salicylic acid).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for pointing that out, Nicodem. Although you have probably done that before, I hadn't appreciated what a useful resource that you have
created.
Further to the OP, extra nitration can give 3,5-Dinitrosalicylic acid, and even very mild reducing agents will reduce that to 3-amino-5-nitrosalicylic acid. That gives you two new
compounds, and one of them is an aromatic amine that might be turned into another azo dye.
Of course, these salicylic derivatives may also be esterified. Methyl salicylate has the interesting smell of Oil of Wintergreen, or Linament.
Imagine what the esters of these derivatives might smell like.
[Edited on 8-12-2013 by Paddywhacker]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
In theory one could make benzene by refluxing salycilic acid with zinc dust. The idea is based on the classical reduction of phenol with zinc dust to
yield benzene and zinc oxide.
Although I would not say aspirins are cheap here, 15$ for about 150 g of active compound.
I never asked for this.
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
The synthesis of aspirin itself is very simple and you would save money by making your own and not buying it at the drugstore.
the procedure:
1.Weigh out 3.0 g of salicylic acid and place in a 250 ml Erlenmeyer flask.
2.Measure out 6.0 ml of acetic anhydride and add this to your flask.
3.Add 5 to 10 drops of 85% phosphoric acid, a catalyst, to the flask and swirl to mix everything thoroughly.
4.Heat the mixture for about 10 min. in a beaker of warm water (70-80 oC).
5.After heating add 20 ml of distilled water and cool in an ice bath. Crystals should fall out of solution immediately, if they don't, induce
crystallization by scratching the inside of the flask.
6.Filter and dry the aspirin.
By making your own aspirin and buying all chemicals new from DudaDiesel and UnitedNuclear you could make ruffly 1050g (assuming 80% yield) for $82.
907g salicylic acid from unitednuclear $60
950ml Phosphoric acid from DudaDiesel $12
950ml Glacial acetic acid from DudaDiesel $10
Plus you are left with extra Phosphoric acid and AA.
hmm... I wonder if one could commercialize this procedure and make money by charging less absurd prices for drugs and by buying chemicals bulk...
ps. my calculations may be off by a huge margin....
http://www.dudadiesel.com/search.php?query=acetic
http://unitednuclear.com/index.php?main_page=product_info&am...
how to make aspirin:
http://wwwchem.csustan.edu/consumer/aspirincons/aspirincons....
http://www.youtube.com/watch?v=amTAuK25P6c
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I just bought 4 bottles of 1000 aspirins for $37.15. That's 1300g of active ingredient. Acetone should be able to separate the active from the
starch binder with just a simple filtration. I would want to do a vacuum distillation just to keep the heat down to keep the salicylic acid molecule
from deacetylating and recycle the acetone.
[Edited on 9-12-2013 by hyfalcon]
[Edited on 9-12-2013 by hyfalcon]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
No. In this particular case it's blindingly obvious that this is wrong (because aspirin is dirt cheap in bulk, in US market at least), but even in
cases where things superficially look more promising, there is always the time value of your own labor, the uncertainty in the outcome, and the
initial investment in getting the reaction to work.
Also, your reaction steps involve acetic anhydride but the price you give is for glacial acetic acid. But it doesn't really matter, it wouldn't be
economical regardless.
The less you bet, the more you lose when you win.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I think the HeyBro's original post was asking what can be done with aspirin not how you make it. You can buy both aspirin and salicylic acid off ebay
for peanuts why waste time making it?
@ HeyBro I suggest you use the search engine and check out the picric acid thread, the sulphosalicylic acid prep tread, Phenol .... etc
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
"@ HeyBro I suggest you use the search engine and check out the picric acid thread, the sulphosalicylic acid prep tread, Phenol .... etc "
the point of this thread was to collectively post experiments (from aspirin and its derivatives ) and bounce ideas from one and other so everyone
could benefit, other wise i would of just searched them and not made a thread.
thanks for the links Nicodem, but i can't access them, safari says the certificate is funny. all of the https SM threads i can't get access to for
some reason. Any way, thanks for awesome ideas so far. my idea is this "aspirin to paracetamol"
1. hydrolyse ASA to yield SA
2. decarboxylate SA to yield phenol
3.nitrate phenol with Sodium nitrate and dilute sulphuric acid
3. extract and purify 4-nitrophenol from reaction mixture ( also containing 2-nitrophenol, among other things)
4. Reduce nitro group to an amine (can't exactly do this step as i don't have a strong enough reducer such as NaBH4)
5. acetylation of 4-aminophenol to form N-(4-hydroxyphenyl)ethanamide aka paracetamol
Of course this is heavily over simplified but it's just my idea for aspirin to paracetamol. This is also just explore some organic synthesis and for
the challenge. Thoughts?
[Edited on 10-12-2013 by HeYBrO]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by HeYBrO  | | thanks for the links Nicodem, but i can't access them, safari says the certificate is funny. all of the https SM threads i can't get access to for
some reason. |
Obviously, like for any HTTPS site, you first need to accept the certificate. By refusing it, you essentially say to your browser that you do not want
to access the site trough the HTTPS protocol (so it refuses you access). I doubt that your "safari says the certificate is funny". That sounds a bit
non-profesional coming from some software. Utmost it says it is self-signed or something like that, which is irrelevant to what you want (access). By
the way, we even have a search engine where you can type in "aspirin".
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Ok, thanks Nicodem.
edit: for my last post here are some references
http://home.comcast.net/~drewpoche/CHEM3/CHEM_III_labs_files...
http://www.rsc.org/learn-chemistry/content/filerepository/CM...
[Edited on 11-12-2013 by HeYBrO]
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
[img][/img]
My proposed ( and simplified) reaction scheme with some products omitted.
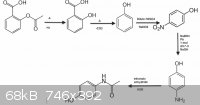
[Edited on 12-12-2013 by HeYBrO]
[Edited on 12-12-2013 by HeYBrO]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A scheme obviously is of no value without references. What is the reference for the nitration? Where did you got that reduction of the nitro group
with NaBH4, and what does Pb do there?
And to complete the irony of the aspirin to paracetamol transformation, make sure you do the N-acetylation with aspirin as the reagent:
https://www.sciencemadness.org/whisper/viewthread.php?tid=11...
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
if looked a little closer, Nicodem, you would see all the relevant information for this scheme including my original idea, which, apparently you
ignored and scolded me for saying "safari says the certificate is funny". here you go, all on one post:
1. hydrolyse ASA to yield SA
2. decarboxylate SA to yield phenol
3.nitrate phenol with Sodium nitrate and dilute sulphuric acid
3. extract and purify 4-nitrophenol from reaction mixture ( also containing 2-nitrophenol, among other things)
4. Reduce nitro group to an amine (can't exactly do this step as i don't have a strong enough reducer such as NaBH4)
5. acetylation of 4-aminophenol to form N-(4-hydroxyphenyl)ethanamide aka paracetamol
references:
http://home.comcast.net/~drewpoche/CHEM3/CHEM_III_labs_files... http://www.rsc.org/learn-chemistry/content/filerepository/CM...
course this is heavily over simplified but it's just my idea for
aspirin to paracetamol. This is also just explore some organic synthesis and for the challenge. Thoughts? feel free to delete my other posts.
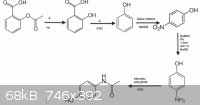
[Edited on 13-12-2013 by HeYBrO]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by HeYBrO  |
3.nitrate phenol with Sodium nitrate and dilute sulphuric acid
3. extract and purify 4-nitrophenol from reaction mixture ( also containing 2-nitrophenol, among other things)
|
'among other things' may be explosive. Ie. picric acid.
Chemistry is never 100% safe but without a more thorough
description of the process (ie. reagent amounts and method)
this could be dangerous for beginners.
Seeing as this is the beginnings forum, a warning is probably in order.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
are serious macckone? if you haven't noticed 95% of the posts on this forum are dangerous and without warning. does no one here read posts to the
end? " course this is heavily over simplified but it's just my idea for aspirin to paracetamol". I'm sorry if this comes off antagonistic, but it
seems generally people like to ignore the chemistry and rather nit pick even if the information is there. I gave my sources, which give adequate
warning about the hazards of Phenol to paracetamol. http://www.rsc.org/learn-chemistry/content/filerepository/CM...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Just a friendly advice: I'm sure you would get less of such replies, if you cited correctly. Just posting two links to be later followed with some
vacant synthetic proposals is not a constructive approach. It seems, I'm not the only one who was mislead by this. You need to connect what you
propose with the reference from where it originates.
By the way, the article says palladium on carbon (Pd-C), not lead (Pb). And it is a catalytic transfer hydrogenation, which means you don't
necessarily need NaBH4. Other hydrogen donors might do. Hydrogen would for sure. Alternatively, you can do either a metal dissolving
reduction of the nitrophenol, or use sodium dithionite. You just need to search the literature for the alternatives.
The mononitration of phenol with nitric acid and then separating the isomers is also not an elegant solution. There are better alternatives.
Supposedly it is possible to oxidize the nitrosated phenol to the p-nitrophenol and this can be done in a one-pot procedure according to
EP0626366 (see US5414148 for the English translation). Such an approach quite cleanly gives p-nitrophenol and in good yields, because the
nitrosation has excellent regioselectivity and gives only the monosubstituted product. The procedure in the examples looks quite straightforward and
give a good quality product. Of course, you can just nitrosate the phenol to the p-quinone monoxime and reduce it to p-aminophenol,
instead of going trough the p-nitrophenol (essentially what the patent describes, except for skipping the oxidation with HNO3, as
is for example described in DOI: 10.5012/bkcs.2010.31.10.3007). However, some member here reported troubles in trying to nirosate phenol, but
apparently it can be done by respecting the critical parameters (UTFSE). The p-quinone monoxime can be reduced by various means,
including directly with NaBH4 (without Pd-C, DOI: 10.1021/ja01495a032) or even by such exotic ways as using baker's yeast (DOI:
10.1016/0040-4039(95)00398-V).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
My apologies for my haphazard posting, indeed i can see how it wasn't transparent. Also i do understand the difference between Pd/Pb i must of not
being paying attention and then i didn't notice.
I corrected it here, is there a way you could replace the old photo with the new as i can't edit it. Thanks for the great resources, i'll check them
out once I'm finished posting.
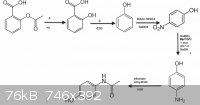
|
|
|
eidolonicaurum
Hazard to Self
 
Posts: 71
Registered: 2-1-2014
Location: Area 51
Member Is Offline
Mood: Hydric
|
|
https://www.youtube.com/watch?v=VTLnNWQhSMI&feature=yout...
Oil of wintergreen prep from aspirin.
|
|
|