| Pages:
1
2
3 |
NiCoLi_BrLiNTe
Harmless

Posts: 18
Registered: 22-5-2013
Location: just outside the walls of Warwick castle
Member Is Offline
Mood: perplexed
|
|
chloroform by the haloform reaction
The following are pictures of the process I used to finally produce chloroform by the haloform reaction. I'm still not really sure what the problem
was in the first place; although I think it may have been overkill on the cooling of reagents (i.e. using cryo-hol to try and keep everything at a
constant -50C or simply the incorrect ratios (although I'm uncertain how this would cause the reaction to stall). Whatever the cause, I
think next time I'll skip over the reflux stage and just go strait to adding the reagents in a distillation setup with vigorous stirring of the
reaction flask.
The dropping funnel was charged with 22 ml of acetone, the pictures are kind of self explanatory, but let me know if theres any part you're especially
interested in, I have video of most of the distillation and will try to explain as best as I can. Finally I know adding the reagents strait into the
distillation apparatus is probably risky, but I believe with a bit of planning and some precautions, it will be a worthwhile way to increase yield and
save time.
Thank you all for your help and suggestions,
NiK
[Edited on 21-7-2013 by NiCoLi_BrLiNTe]

|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Trichloromethane from bleach and acetone.
Trichloromethane
is prepared from household bleach and pure acetone nail polish remover.
The reaction that takes place is very exothermic, and this rise in heat
must be accounted for, especially when using larger amounts of reagents
and scaling up. I use a salt/ice bath and aggressive magnetic stirring.
Do this under a well ventilated hood or in a ventilated area, because
the acetone and chloroform are volatile and not especially good for you.
The rapid rise in heat during the reaction also makes it possible for
the volatiles to boil out of solution.
Reagents Used:
500ml 8.25% bleach (sodium hypochlorite in alkaline solution.)
12ml Acetone.
Lots of Ice and salt.
Here are some pictures of the process.
Extra strength bleach
 
Pure acetone

500ml of bleach chilled to ~ -12°C

12ml acetone, chilled.

Acetone added with a sep. funnel drop-wise. Followed bbarts advice on allowing a top layer of acetone to form. Mag stirring at full speed at this
point.

Last of the acetone is added.

Cloudiness and exotherm started.

Solution rose in temp to ~ 3°C with aggressive cooling and slow addition.

Solution losses its yellow color and temperature rise stops.

[Edited on 24-7-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
A blob of chloroform seen at the bottom of the flask after 1 hour from first acetone addition.

Sep funnel shots.
 
Chloroform separated from the funnel.

Yeild ~10ml crude chloroform.

I did not do a distillation of the obtained, crude trichloromethane, so it likely contains some dissolved acetone. boiling point measured at ~ 60.5
°C.
Thanks for watching. Any comments, corrections or flaming welcome.
[Edited on 24-7-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That looks good - a very simple procedure with easily attainable reagents. Lots of good pictures. 10mL chloroform on 12mL acetone seems like a very
good yield. I would like to see what the yield is after purification, ie, distillation.
I made chloroform some time ago using the procedure in Brewster (1960) at 1/2 scale. This procedure calls for 100g of HTH calcium hypochlorite and
37mL of acetone. Equipment is a 1000 mL RBF with a reflux condenser. Workup was by steam distillation followed by simple distillation. I combined 3
each 1/2 batches and after the final distillation had just 25mL chloroform on 55.5mL of acetone.
[Edited on 25-7-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Sorry, I somehow missed your respone Magpie.
I thought the crude yield looked to good to be true. Unfourtunatly, my last 14/20 condensor broke months ago (hope Dr. B has some left, when I have
the $...).
This has left me with only a 24/40 option. This would make the mechanical losses quite high I fear. I could add a few jugs of bleach to the ol'
grocery list in a couple weeks and do several, similarly scaled and cooled reactions in sequence and collect the nonpolars together for a 24/40 run.
Im worried that x5ing the scale would make for serious challenges with exotherm.
I vaguely remember reading something about up to 20% acetone impurity can be hard to remove. I need to ustfs real quick to find it.
I'll try and get a more precise yeild data posted soon.
Thank you for the interests. I actually find trichloromethane pretty useful as a solvent, when I have it handy at least.
[Edited on 28-8-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
Chloroform precipitate and invisible chloroform?
i have longer time ago succesfully made a few mL of chloroform using store bought bleach and a small amount of acetone, it gave a large amount of
white fluffy like precipitate, but below all of this there was a very heavy liquid which seemed to be chloroform
now again i have tried with some homemade bleach made from salt water electrolysis for roughly 12 hours, i let the thing run with 5V 40A power supply,
on average this thing can put out 150g NaClO3 after 3 days runtime when i just let the solution cool to room temperature, but the bleach solution i
ended up with im not sure about, but it smelt decently strong
approx 1.5L of bleach solution, homemade, cooled with icecubes had 20mL of acetone added to it and the lid was fitted onto it, it was given a shake
and let stand
after a while a white cloudy layer formed at the top, and later on it settled down (3 hours?)
i have before taken this strange powder and dried out, to then test it for melting point.. it was above 200*C if it even has one
i have read about a precipitate with an existing melting point when this is done with Ca(ClO)2 and acetone but this is not the case when using NaClO
i saw a video where one guy used 400 mL bleach solution and 20 mL acetone, so i suppose 20 mL acetone is not too much when having 1.5L? ive thought
about whether the acetone could dissolve the chloroform, where the acetone would be soluble in water and thus making the chloroform soluble aswell
i see small bubbles clinging to the side of the jar, and when i shake it the chloroform smell gets pretty intense
could it be my acetone had some crap added to prevent chloroform synthesis??
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I haven't heard of adding things to acetone to make it unsuitable for simple chloroform synthesis. Have you used that acetone for anything else?
You might want to distill it just to be safe.
My guess to your problem is just because you stoichoimetry was to far off. You definitely should evaluate the concentration of your NaOCl (aq).
About the precipitate, have you tested it at all yet? Check it's solubility in water, whether it burns in air (indicating an organic compound),
melting point, maybe even boiling point.
|
|
|
Nicodem
|
Threads Merged
11-6-2014 at 07:42 |
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
yeah i have
used it for dissolving organics mostly, had alot of uses for it but its like i dont recall them, strange?
anyways the datasheet from the manufacturer says its pretty high quality, it leaves nothing behind when let evaporate off, its perfectly clear and
burns very well
but yes the stoichiometry might be why, ill let my cell run during the night aswell to get a very high concentration, hopefully..
about the % of the acetone im fairly certain its at least 95%, but the NaClO's concentration i cant tell and i dont know how i could possibly
accurately titrate it?? reacting it with more or less anything is like dumping several fuels oxidizers and catalysts together and burning it off.. if
you have a suggestion for NaClO titration please share
actually i see its possible to do with thiosulfate, but i used the last i had long time ago in an desperate attempt on creating manganese sulfate
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
What about using hydrogen peroxide to test the conc.
the reaction is: H2O2 + NaOCl → NaCl + H2O + O2. So if you use in excess of peroxide, you can just
measure the oxygen generated to get the conc. pretty accurate.
[Edited on 13-6-2014 by Zyklonb]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
1mL 1% i recall to give 3.33mL O2
ill need to first run a quick gas titration of my H2O2 first, estimated to be 12%
no problem, ill try it soon
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
my 12% H2O2 seems to be... 28.5%?!
if 1 mL 1% H2O2 yields 3.33mL of gas
then 1 mL 12% H2O2 should at most generate 39.9 mL gas
but i got 95 mL
i used a 1 mL pipette to drop the H2O2 into a 250 mL flask, coupled up to a ground glass flask adapter to silicone hose, then to 100 mL graduated
cylinder filled with water under water
it was as if i could just keep adding bleach, but after about ... 40 mL it stopped giving off gas
i decided to try and find out what % my H2O2 has simply using excess NaClO solution
anyhow, i seems to get the result that i need about 40 mL bleach solution for 1 mL H2O2
titration with MnO2 gives me 65 mL of gas per mL H2O2
[Edited on 21-6-2014 by Antiswat]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  | my 12% H2O2 seems to be... 28.5%?!
if 1 mL 1% H2O2 yields 3.33mL of gas
[...]
titration with MnO2 gives me 65 mL of gas per mL H2O2
|
I don't know how you arrive at these numbers.
1 ml of 1 w% H2O2 is 0.01 g of pure H2O2.
Assume oxidation as:
H2O2 === > O2 + 2 H+ + 2 e-, so 1 mol of O2 per mol of H2O2, 24.5 L O2 per mol O2 at STP.
So 0.01 g H2O2 / 34 g/mol H2O2 x 24.5 L/mol = 0.720 L = 72 ml.
Not 3.3 ml. The 72 ml corresponds roughly with your MnO2 result.
Gazometer measurements can be tricky and imprecise: try iodometry instead; hydrogen peroxide oxidises iodide to iodine, then titrate iodine with
thiosulphate and starch indicator.
[Edited on 22-6-2014 by blogfast25]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yesterday, I performed the haloform reaction with 600 mL 8.25% NaClO and 15 mL acetone. The reaction proceeded as expected, with an exotherm raising
the temperature to 30 C despite external cooling. However, I have a cloudy emulsion of chloroform that refuses to separate (even after 24h in the
funnel). How could I recover the chloroform?
Ideas I have:
1) Distillation - it is a heterogeneous mixture, so the steam distillation concept applies.
2) Adding a salt to break the emulsion - not sure if this would work.
3) Extracting with a high-boiling nonpolar solvent (xylene) and then distilling.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I've decided to extract with 2x20ml xylene. The extraction went very slowly, but the layers did separate. I think there was some kind of surfactant in
the bleach. I will soon distill the organic layer.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
but...
if 1 mL 1% H2O2 will yield 72 mL of oxygen by being reacted with MnO2
and i used 1 mL 12% H2O2, then it would yield 12 times as being as 72 mL -- being 864 mL of gas
also i got the numbers from another thread
i looked through it to see if anybody would point out that 3.3mL would be wrong, but nobody did so.. which is unusual for false calculations
but 72 mL oxygen per 1% 1mL H2O2 is just unreal
i have found some place that 12% H2O2 should expand 40 times as in oxygen per volume
meaning 1 mL should give 40 mL of oxygen --- 39.9 mL
http://puu.sh/9JPEN.png
a thing i have considered whether could have fucked up my gas titration was that NaClO + H2O2 seemingly generates mono-oxygen (which has some specific
name i ofcourse forgot)
this could potentially account for why i got so much more oxygen than i supposed i would get
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The xylene distillation yielded 4.3 mL chloroform.
I tried the same reaction with a different brand of bleach. No emulsion formed, and the yield was 73% (50.5 mL chloroform (after distillation) from 63
mL acetone).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  | but...
if 1 mL 1% H2O2 will yield 72 mL of oxygen by being reacted with MnO2
and i used 1 mL 12% H2O2, then it would yield 12 times as being as 72 mL -- being 864 mL of gas
also i got the numbers from another thread
i looked through it to see if anybody would point out that 3.3mL would be wrong, but nobody did so.. which is unusual for false calculations
but 72 mL oxygen per 1% 1mL H2O2 is just unreal
i have found some place that 12% H2O2 should expand 40 times as in oxygen per volume
meaning 1 mL should give 40 mL of oxygen --- 39.9 mL
http://puu.sh/9JPEN.png
a thing i have considered whether could have fucked up my gas titration was that NaClO + H2O2 seemingly generates mono-oxygen (which has some specific
name i ofcourse forgot)
this could potentially account for why i got so much more oxygen than i supposed i would get |
Stop getting these basic date from 'another thread' and do the basic calculations yourself.
Mono-oxygen? Atomic oxygen IMMEDIATELY combines to 'normal' diatomic oxygen.
"but 72 mL oxygen per 1% 1mL H2O2 is just unreal"
Why is it 'unreal'? And what does 'unreal' mean in your strange little world?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Assume oxidation as:
H2O2 === > O2 + 2 H+ + 2 e-, so 1 mol of O2 per mol of H2O2, 24.5 L O2 per mol O2 at STP.
So 0.01 g H2O2 / 34 g/mol H2O2 x 24.5 L/mol = 0.720 L = 72 ml. |
The reaction equation is only valid for the H2O2 oxydation reactions (like with NaOCl), but not also for the disproportionations
(like with MnO2 or iodide catalysis) where the ratio is 2H2O2 -> O2.
Besides, the result of your calculation is 12.25 mL (at least according to my calculator).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
true, the calculations i found was somehow incorrect, found 12% H2O2 to must yield 41.4 mL oxygen using MnO2 to catalyse it into free oxygen
recalling 40 mL homemade NaClO solution was used to fully react 1mL 12% H2O2 it would yield me somewhat about 0.65% NaClO solution which seems absurd
perhaps i should try electrolysing a NaCl solution well cooled down for longer time until i reach less than 10 mL NaClO solution per mL of H2O2 needed
to react it fully
but still doesnt make up for white precipitate, in which seems not to occur when using store bought NaClO solution
|
|
|
Aquakidney
Harmless

Posts: 5
Registered: 1-9-2014
Location: The High Desert
Member Is Offline
Mood: No Mood
|
|
Hello,
Long-time lurker, first-time poster. I was doing this same prep a few months ago and found this procedure online for determining OCl-
concentrations. Does require thiosulfate, but getting some would be a lot less trouble than trying to collect and measure a gas.
Incidentally, my particular lot of Clorox Concentrated Bleach actually contained 8.62% NaOCl, not the advertised 8.25%, so that was nice.
The next project is 2,4-dinitrophenylhydrazine to see if there's any acetone left in the CHCl3.
Attachment: hypochlorite lab.pdf (57kB)
This file has been downloaded 2014 times
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
inverted measuring cylinder filled with water in water, its not that bad, but you will probably get wet hands somehow
supposing the too high concentration may be because they expect it to decompose, in which it ofcourse does over time
anyhow, happy first post and welcome to sciencemadness
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Attachment: Was Chloroform produced before 1831.pdf (89kB)
This file has been downloaded 1155 times
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
On somewhat larger scale
Did this preparation on a larger scale, since I want to do some experiments using chloroform as a solvent. Yield suffered somewhat.
Experimental
Four containers of 8.25% sodium hypochlorite bleach (Chlorox), each containing 3.78 L of liquid, were chilled for four hours in a freezer, at which
point some ice began forming. Estimated average temperature was slightly below freezing.
The contents were poured in to a 23 L glass carboy.

Empties
342g of acetone at room temperature were measured in to a 500ml beaker and then poured in to the carboy. The acetone remained on top of the bleach,
forming a separate layer that gradually turned yellow. The carboy was repeatedly tipped and rotated in order to agitate the reaction mixture and mix
the layers. After a few minutes, the upper part of the mixture grew warm and very small droplets of chloroform began to fall through the solution and
accumulate at the bottom of the carboy.

The yellow layer
After repeated swirling (which should be done with caution if you are using a large glass carboy, as breakage can lead to deep lacerations), the top
layer became mixed with the remainder of the solution. The carboy was left to stand overnight so as to allow the reaction to finish, and any suspended
droplets of chloroform to settle to the bottom. Subsequently the top aqueous solution was decanted to the extent possible (without loss of the bottom
layer), the remaining 750 ml or so poured via a funnel in to another narrow-mouth glass container, some further top layer removed by decantation and
the remainder poured in to a 500ml separatory funnel:

Separation
Following separation the chloroform was weighed, yielding 401g of crude product (57% yield based on acetone).
The chloroform was then stored for several days in a screw top container, which (due to inadequate closure and evaporation) resulted in the loss of
15g of chloroform on re-weighing. Subsequently, the chloroform was stored overnight with an equal weight of 95% sulfuric acid, after which the
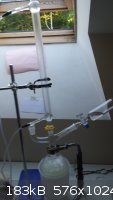
sulfuric acid and chloroform were poured in to a 1L round flask with flat bottom and set up for distillation (yes, I know that's not supposed to be a
Hempel column in the middle there...):

Discolored acid

Misuse of Hempel column
The sulfuric acid darkened noticeably as a result of this (it had been a very light amber color). Though it should not react with chloroform to an
appreciable extent, I assume that impurities in the chloroform formed condensation products that led to this discoloration.
Distillation yielded approximately 235ml of chloroform, a yield of about 50% (I haven't weighed it yet).

Nearly full
This is a slightly disappointing yield. Previous runs on a smaller scale have yielded up to 75% or so. I would assume that the difficulties in mixing
the acetone with the bleach when using vessels of this size may result in more extended contact between chloroform and acetone, resulting in more
losses to the chlorobutanol side reaction. Adding the acetone in portions or else using some kind of mechanical stirring might mitigate this.
The less you bet, the more you lose when you win.
|
|
|
| Pages:
1
2
3 |