CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
N-Acetylation of taurine
Heres taurine:

and heres acamprosate, its acetylated analogue:
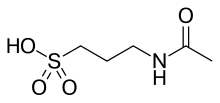
From what I read, esters can be used instead of acid anhydrides or acyl halides for N-acylations. So if one were to reflux a solution of taurine in
ethyl acetate, would acamprosate and ethanol be produced?
|
|
|
Prometheus23
Hazard to Self
 
Posts: 62
Registered: 6-6-2012
Member Is Offline
Mood: No Mood
|
|
Esters can indeed be used to n-acylate amines. Ethyl acetate would probably work fine, and even esters like aspirin can sometimes be used.
The only probably is that acamprosate is not n-acetyltaurine but n-acetylhomotaurine. So you would need to use homotaurine instead of taurine for the
synthesis.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Taurine, however, is not HOS(O)2CH2CH2NH2 (as you've drawn it), but the zwitterion
-O3SCH2CH2NH3+, with a negative charge on the sulphate end and a positive charge on
the nitrogen. I expect that you'd need to deprotonate the nitrogen before it reacted with the ester.
[Edited on 12-4-2013 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I wonder if ethyl acetate would be likely to acetlyate the nitrogen or ethylate the sulphonic acid.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you had the anion, the nitrogen would be far more nucleophilic than the sulphate end. I don't think the acetyl-sulphate compound would form (and
if it did, it would be very reactive towards nucleophiles).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Prometheus23
Hazard to Self
 
Posts: 62
Registered: 6-6-2012
Member Is Offline
Mood: No Mood
|
|
Yes the taurine or homotaurine would obviously need to be in the anionic state to react with ethyl acetate. And I agree with DraconicAcid, I don't
think the sulfonate group would react with the ethyl acetate to any appreciable amount.
|
|
|
CrimpJiggler
Hazard to Self
 
Posts: 75
Registered: 1-9-2011
Member Is Offline
Mood: No Mood
|
|
Whoops, I didn't notice that extra carbon in acamprosate. Homotaurine isn't as readily available as taurine.
|
|
|
Nicodem
|
Thread Moved
13-4-2013 at 00:00 |
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Homotaurine is cheaply and readily made;
http://www.nrcresearchpress.com/doi/pdf/10.1139/v62-339
|
|
|