| Pages:
1
2 |
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
potassium nitrate and copper sulphate
K(NO3)2 + CuSO4 = KSO4 + Cu(NO3)2
Would this present any problems in also making copper nitrate. I know I'm ignorant, but would the right substance precipitate and would there be any
hazards?
Trying to balance this stoichemetry speaking is a problem because I simply do not know what figures to use due to the fact that both substances have
different g/mol figures for different hydrated states, and since I know nothing about how things react I do not know which figures to use. The
copper nitrate would of course be any hydrated state and the copper sulphate, if it precipitates then that would have a pentehydrate state. But yep,
I'm without expertise in this.
Forgot to add that my copper nitrate via the Nitric acid coming along nicely, but since I have these chemicals also I thought I would try them out,
for both practical reasons and learning.
[Edited on 1-10-2012 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Yes, this could be problematic as both copper (II) nitrate and potassium sulfate are soluble in water, quite well.
Also, note that the formula of potassium sulfate is K2SO4 as the potassium cation is monovalent where as the sulfate anion is divalent, and the
formula of potassium nitrate is KNO3 as both the ions are monovalent.
The overall equation is; 2KNO3 + CuSO4 → K2SO4 + Cu(NO3)2, but you will, of course, not just get copper nitrate as the product. What actually
happens is;
2[K+] + 2[NO3-] + [Cu2+] + [SO42-) → 2[K+] + [SO42-) + [Cu2+] + 2[NO3-]
Thus, this cannot possibly be a double-displacement reaction as the chemistry is done in aqueous solution with all products soluble.
Dissolving copper in nitric acid is a better choice, as is using a nitrate salt where the cation has an insoluble sulfate, e.g. calcium, strontium and
barium nitrates. Note that magnesium nitrate cannot be used as the carbonate does not fit the trend and is soluble.
[Edited on 1-10-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
I doubt it would work. The solubility for copper sulfate is the lowest, and that of copper nitrate is the highest. Most interestingly, you might be
able to make a double salt, but it won't be simple to purify into either nitrate. If you must make it using copper sulfate, use calcium, strontium, or
barium nitrates. Barium has the least soluble sulfate of the three, but is quite toxic. Note that magnesium does not have an insoluble sulfate, and
has a higher solubility than copper at 20C.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Hexavalent, Vargouille, thanks. I had read about calcium nitrate being used but do not have any. Now I understand why the calcium and Barium
nitrates are recommended. Off to read now about divalent and monovalent cations.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
No. Potassium sulfate is the least soluble and would precipitate first, especially at high temperatures (e.g, as you boil off water) as its solubility
does not rise as rapidly as that of copper nitrate (or copper sulfate, for that matter) with increasing temperature.
You could proceed as follows:
Dissolve four moles (404g) of KNO3 in a liter of warm water
Dissolve two moles (499g) of CuSO4 (pentahydrate) in a liter of hot water
Mix these, boil off 1500g of water (you would likely need to filter or decant at times to get rid of the precipitating K2SO4, or deal with a lot of
bumping...). Cool to freezing, filter to remove more K2SO4. The remaining liquid should still contain all the copper and nitrate, but less than 40g of
K2SO4 (the remaining 310g of it having precipitated). You could then drive off the remaining water (carefully, so as not to decompose the copper
nitrate) and have ~90% pure Cu(NO<sub>3</sub> <sub>2</sub>
with the remainder as potassium sulfate. Alternatively, adding 200g of absolute ethanol would precipitate 90% of the remaining K2SO4 and you could
then obtain 98-99% pure copper nitrate. <sub>2</sub>
with the remainder as potassium sulfate. Alternatively, adding 200g of absolute ethanol would precipitate 90% of the remaining K2SO4 and you could
then obtain 98-99% pure copper nitrate.
The less you bet, the more you lose when you win.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Hi bbarthlog, interesting, as an exercise it would be interesting, though for practical reasons I would never want to make the nitrate this way being
that something always goes wrong when I go for lon-winded processes. But by way of a learning exercise I might try this one out later.
[Edited on 2-10-2012 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
My apologies, I must have misread the solubility statistics for potassium sulfate. In any case, I find the procedure somewhat spurious. I should think
that, considering the nature of ionic compounds, copper nitrate would decompose at boiling temperatures. Have you tried this procedure before?
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I have always wondered about the decomposition of copper nitrate - I once had a solution of copper nitrate that I evaporated....it absolutely reeked.
The smell hit the back of your throat and was unbearable. At first, I thought it was excess nitric acid but now, looking back, it could be the
decomp. of the nitrate.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
this is true potassium and copper form a double sulfate,the hydrate of which is a beautiful sky blue. I prepared this once by accident and posted in
the pretty pictures thread: http://www.sciencemadness.org/talk/viewthread.php?tid=14644&...
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Out of curiosity, what is the fascination with the decomposition of copper nitrate?
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | | I have always wondered about the decomposition of copper nitrate - I once had a solution of copper nitrate that I evaporated....it absolutely reeked.
The smell hit the back of your throat and was unbearable. At first, I thought it was excess nitric acid but now, looking back, it could be the
decomp. of the nitrate. |
Per Wiki (http://en.wikipedia.org/wiki/Cu(NO3)2 ), one cannot dehydrate the Cu(NO3)2.3H2O. To quote:
"Attempted dehydration of any of the hydrated copper(II) nitrates by heating instead affords the oxides, not Cu(NO3)2. At 80 °C, the hydrates convert
to "basic copper nitrate" (Cu2(NO3)(OH)3), which converts to CuO at 180 °C.[2] Exploiting this reactivity, copper nitrate can be used to generate
nitric acid by heating it until decomposition and passing the fumes directly into water. This method is similar to the last step in the Ostwald
process. The equations are as follows:
2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2
3NO2 + H2O → 2HNO3 + NO "
So the comment that the reaction is completely ionic with no reaction is not entirely correct as upon removing the water, the decomposition of the
Cu(NO3)2 moves the reaction to the right with absolutely no Copper nitrate remaining.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Vargouille  | | ...I should think that, considering the nature of ionic compounds, copper nitrate would decompose at boiling temperatures. Have you tried this
procedure before? |
No, it is entirely speculative. Given the existence of a double salt, it seems unlikely to work, and I can certainly believe that copper nitrate
undergoes some slight decomposition at boiling temperatures, giving off NOx gases. But my CRC Handbook gives a decomposition temperature of 170C.
The less you bet, the more you lose when you win.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bbartlog  | | No, it is entirely speculative. Given the existence of a double salt, it seems unlikely to work, and I can certainly believe that copper nitrate
undergoes some slight decomposition at boiling temperatures, giving off NOx gases. But my CRC Handbook gives a decomposition temperature of 170C.
|
My CRC Handbook says the same, yet the Wiki article you linked claims a decomposition temperature of 80C. A "Handbook of Copper Compounds and Applications" states that basic copper nitrate is formed by careful thermal decomposition of the trihydrate or
"by direct hydrolysis from the solution with alkali" (Pg. 84). The thermal decomposition mechanism is on the same page, which does put some doubt on
the Wikipedia claim of decomposition at 80C, yet Ullmann's Encyclopedia of Industrial Chemistry says:
| Quote: | | The anhydrous nitrate is not produced by heating the hydrates; instead, decomposition to the basic copper(II) nitrate [12158-75-7] , Cu2(NO3)(OH)3 ,
begins around 80 °C. Conversion to copper(II) oxide is complete at 180 °C. |
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Another path, treat aqueous Ca(NO3)2 with Oxalic acid to produce dilute HNO3 and the highly insoluble Calcium oxalate salt. Reaction:
Ca(NO3)2 (aq) + H2C2O4 (aq) --> CaC2O4 (s) + 2 HNO3 (l)
For details/precautions see http://www.sciencemadness.org/talk/viewthread.php?tid=18963 . Note, many Oxalates are insoluble so this synthesis can produce acids of high
purity, but producing concentrated acids is often more difficult and potentially dangerous (for example, reaction with a chlorate salt will form
Chloric acid and also could reduce it to explosive ClO2, see http://www.sciencemadness.org/talk/viewthread.php?tid=18963&... , a violent ejection has also been reported on attempting to form concentrated
H2SO4).
Decant and react the dilute HNO3 with Cu to form NO to which I would combine with air (from an air pump) to form NO2 which you dissolve in water.
Reactions:
3Cu(s) + 8HNO3(aq) ——> 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
Reference/video: http://www.angelo.edu/faculty/kboudrea/demos/copper_HNO3/Cu_...
2 NO + O2 --> 2 NO2
3 NO2 + H2O --> 2 HNO3 + NO
[Edited on 3-10-2012 by AJKOER]
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
chris, you're interested in the anhydrous salt right?
http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@ter...
| Quote: |
Methods of Manufacturing:
... Sublimation of copper(II) nitrate under vacuum from ... mixt of copper(II) bromide and silver nitrate at about 200 deg C. ...[Kirk-Othmer
Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V7 (1982) 105] **PEER REVIEWED**
|
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Just curious, if you heated the CuSO4 to 150 Degrees
Celcium, until it melt, and then put the KNO3 inside the
liquid CuSO4 would the reaction work? Of course its just
an imaginary situation, since I guess the Cu(NO3)2 has
a quite low decomposition temperature, so controlling
the reaction temperature would be really hard. Maybe if
that was possible it would be a way to get anhydrous
Cu(NO3)2, right?
I might be tripping here 
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by tetrahedron  | chris, you're interested in the anhydrous salt right?
http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@ter...
| Quote: |
Methods of Manufacturing:
... Sublimation of copper(II) nitrate under vacuum from ... mixt of copper(II) bromide and silver nitrate at about 200 deg C. ...[Kirk-Othmer
Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V7 (1982) 105] **PEER REVIEWED**
|
|
Thanks, but no, no need for the anhydrous, besides, far too complicated to do at home.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
For the record, supposedly one can convert any metal hydrate via Thionyl chloride (SOCl2) to the anhydrous salt. To quote from Wikipedia (http://en.wikipedia.org/wiki/Thionyl_chloride ):
"Anhydrous metal chlorides may be obtained from hydrated metal chlorides by refluxing in freshly distilled thionyl chloride:[14]
MCln·xH2O + x SOCl2 → MCln + x SO2 + 2x HCl "
One problem is, of course, one must have (or be able to prepare) "freshly distilled thionyl chloride".
Perhaps per Wikipedia (http://en.wikipedia.org/wiki/SCl2 ):
"SCl2 is produced by the chlorination of either elemental sulfur or disulfur dichloride.[2] The process occurs in a series of steps, some of which
are:
S8 + 4 Cl2 → 4 S2Cl2; ΔH = −58.2 kJ/mol
S2Cl2 + Cl2 → 2 SCl2; ΔH = −40.6 kJ/mol "
Followed by:
SO2 + Cl2 + SCl2 → 2 SOCl2
to create the Thionyl chloride, obviously not especially easy.
[Edited on 3-10-2012 by AJKOER]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Wikipedia and everything else I've seen only suggests this method as general for chlorides. I'm not necessarily saying it won't work... but it
wouldn't surprise me if (in the case of copper nitrate) you ended up liberating NOx and producing CuCl2, or somesuch.
The less you bet, the more you lose when you win.
|
|
|
semiconductive
Hazard to Others
  
Posts: 326
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by bbartlog  |
No. Potassium sulfate is the least soluble and would precipitate first, especially at high temperatures (e.g, as you boil off water) as its solubility
does not rise as rapidly as that of copper nitrate (or copper sulfate, for that matter) with increasing temperature.
You could proceed as follows:
Dissolve four moles (404g) of KNO3 in a liter of warm water
Dissolve two moles (499g) of CuSO4 (pentahydrate) in a liter of hot water
Mix these, boil off 1500g of water (you would likely need to filter or decant at times to get rid of the precipitating K2SO4, or deal with a lot of
bumping...). Cool to freezing, filter to remove more K2SO4. The remaining liquid should still contain all the copper and nitrate, but less than 40g of
K2SO4 (the remaining 310g of it having precipitated). You could then drive off the remaining water (carefully, so as not to decompose the copper
nitrate) and have ~90% pure Cu(NO<sub>3</sub> <sub>2</sub>
with the remainder as potassium sulfate. Alternatively, adding 200g of absolute ethanol would precipitate 90% of the remaining K2SO4 and you could
then obtain 98-99% pure copper nitrate. <sub>2</sub>
with the remainder as potassium sulfate. Alternatively, adding 200g of absolute ethanol would precipitate 90% of the remaining K2SO4 and you could
then obtain 98-99% pure copper nitrate.
|
Tried this at 1/100 scale, 4g KNO3 (stump burner) 5g CuSO4.5H2O (root killer);
20mL of water.
Result of first attempt ... reddish brown sludge on bottom of flask as soon as water got to boiling, lots of fizzing and splashing with choking smell
that attacked back of throat.
I capped flask immediately, and reduced heat. Under vaccum condition, boiling is now proceeding in a reflux situation stably. Red brown sludge is
not dissolving or turning white (color of potassium sulfate.)
This is consistent with later posts claiming that decomposition of copper nitrate produces that smell; and wikipedia stating that decompostiion occurs
at 80C and above. 
When I allow boiling to stop, color of solution is blue (not green as in photo).
I Will retry experiment with 99% Isopropyl alcohol to see if reaction will carry out slowly with only a little water present and desired product
being dissolved in alcohol at low heat.
Edit: In first photo, I have added an extra 20ml of water when trying to reduce loss of nitrate.
Second photo is after boiling over-night. The brown sludge has all turned to pretty copper metal. The flask has been boiling under a partial vaccuum
as the heat was turned down after stoppering the flask; so little to no air / oxygen from outside got in. It's difficult to see copper color through
the solution; the color in second picture is now the true color of the solution.
 
[Edited on 26-1-2018 by semiconductive]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Just use calcium nitrate and copper sulfate! Calcium sulfate precipitates quantitatively with none of this boiling silliness, and
calcium is one of the cheapest nitrates to get.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by Hexavalent  | | I have always wondered about the decomposition of copper nitrate - I once had a solution of copper nitrate that I evaporated....it absolutely reeked.
The smell hit the back of your throat and was unbearable. At first, I thought it was excess nitric acid but now, looking back, it could be the
decomp. of the nitrate. |
I’ve never had any issues with decomposition when evaporating down copper nitrate solutions, definitely no strange odours, or anything untoward for
that matter. Exactly the same as how you would prepare copper sulphate crystals. Or are you referring to evaporation at elevated temperatures? I know
that the nitrate begins to lose water around room temperature, but it’s not until well over 100C that the nitrate itself breaks down..
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by semiconductive  |
This is consistent with later posts claiming that decomposition of copper nitrate produces that smell; and wikipedia stating that decompostiion occurs
at 80C and above. 
|
Well there’s a reason why we shouldn’t trust Wikipedia, I’ve got a completely different set of values. When I redissolve my salts, including
copper nitrate, I always heat to boiling and have had no issue with decomposition below 100C. Maybe there are impurities affecting the stability?
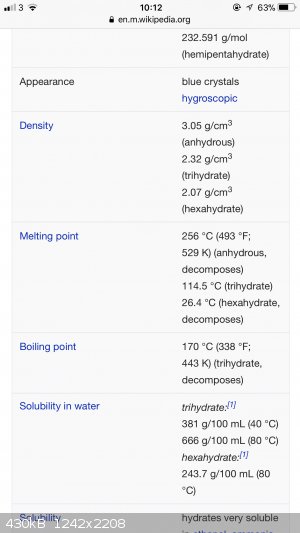
[Edited on 26-1-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A word of caution with respect to avoiding iron impurities and air.
For example, using tap water containing ferrous bicarbonate implies that some soluble Cu(ll) will be converted into Cu(l) via the equilibrium reaction
(referred to as a redox couple):
Cu(ll) + Fe(ll) = Cu(l) + Fe(lll)
Even if the Cu(l) is soluble (by forming a complex), further problems via:
Cu(l) (aq) + O2 (from boiling in air) + 2 H+ --> Cu(OH)2 (s)
which has an electrochemical basis. This leads to the creation of basic salts, or in the current context, basic copper nitrate.
See my prior comments and links at http://www.sciencemadness.org/talk/viewthread.php?tid=77903#... .
[Edited on 26-1-2018 by AJKOER]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Uh, no. People eat BaSO4 every day, as an X-ray contrast for the GI tract.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
| Pages:
1
2 |