IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Ester nomenclature being used wrong?
Hey guys,
My question might sound a little bit stupid, or maybe not..
I know that Esters are results of a reaction involving an
organic acid and an organic alcohol. It's a organic group
with the following structure:
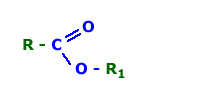
But I'm reading a book which uses the name "ester" in some
compounds that doesnt have this structure. For example the
structure in the attachment is called β-nitro-nitrate ester.
Isn't that wrong? Is it like some "common but wrong"
nomenclature for a product derivated from an acid and an
alcohol reaction?
Was just curious about that.

|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Check the english wiki artical for a good explanation. An organic or inorganic acid... at least one -OH group replaced, etc, etc.
[Edited on 2-10-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
The first definition it gave:
"Esters are chemical compounds consisting of a carbonyl adjacent to an ether linkage. They are derived by reacting an oxoacid with a
hydroxyl compound such as an alcohol or phenol."
However I found something interesting on the same page.
"Inorganic esters" "Ester is a general term for the product derived from the condensation of an acid and an alcohol. Thus, the nomenclature
extends to inorganic oxo acids"
That means that I can use the term ester to a product that comes from an alcohol and an inorganic acid as long as its an oxoacid? Would it be right to
use it just as ruled by IUPAC names?
I know it sound a little stupid, but I guess this nomenclature isnt really diffunded here. The page on wikipedia that talks about Ester in my language
doesnt mention "inorganic esters" and even my organic chemistry teacher thought it quite wierd to use ester in this sort of structure..
But nice to know this other usage of its nomenclature 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IanCaio  | The first definition it gave:
"Esters are chemical compounds consisting of a carbonyl adjacent to an ether linkage. They are derived by reacting an oxoacid with a
hydroxyl compound such as an alcohol or phenol." |
That is a totally wrong definition for esters. Esters have nothing to do with the carbonyl group directly, unless they are carboxylic, carbonic or
carbamic acids esters. And even in that case the presence of the carbonyl group is circumstantial. Therefore, its presence can not be part of the
definition.
Besides, why do you check for the definition on secondary sources, such as Wikipedia, when the IUPAC's Gold book is freely available via their
website:
| Quote: |
esters
Compounds formally derived from an oxoacid RkE(=O)l(OH)m, (l ≠ 0) and an alcohol, phenol, heteroarenol, or enol
by linking with formal loss of water from an acidic hydroxy group of the former and a hydroxy group of the latter. By extension acyl derivatives of
alcohols, etc. Acyl derivatives of chalcogen analogues of alcohols (thiols, selenols, tellurols) etc. are included. E.g. R'C(=O)(OR) , R'C(=S)(OR) ,
R'C(=O)(SR) , R'S(=O)2(OR) , (HO)2P(=O)(OR) , (R'S)2C(=O) , ROCN (but not R–NCO ) (R ≠ H).
Note:
O-Alkyl derivatives of other acidic compounds [see amides (1)] may be named as esters but do not belong to the class esters proper. E.g. (Ph)2POCH3
methyl diphenylphosphinite.
cited from the IUPAC Gold Book |
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
IanCaio
Hazard to Self
 
Posts: 52
Registered: 26-9-2012
Member Is Offline
Mood: No Mood
|
|
Thanks a lot Nicodem,
I never actually took a look at this Gold Book, sounds perfect
for any kind of doubt about organic nomenclature. For sure
a much more trustable source than other websites.
I understand now then, esters are only products from a reaction
that liberates water, from an oxoacid (which can be organic or
inorganic) and usually an alcohol (but it can happen with other
types of compounds too).
This Gold book is gonna be my main reference now on 
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
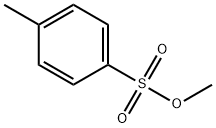
Sometimes people even refer to alkyl tosylates as 'tosylate esters'
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Which is natural, considering it is an ester of tosylic acid.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
triplepoint
Hazard to Others
  
Posts: 127
Registered: 11-4-2012
Location: U.S.
Member Is Offline
Mood: in equilibrium
|
|
Don't be so sad. Just work hard and be a good boy and we may let you in some day.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Or, if you have oil, just wait.
On a slightly more serious note, do some of those 95% or so know that words are not used "wrong" they are sometimes used "wrongly".
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
On this side of the lake, we usually say "used incorrectly". The word "wrongly" is seldom used at all.
Only common example I can think of is "Wrongly accused".
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
I don't really see how this is relevant to this topic. Anyways, it's a hint to USA-centered members to remember that not everyone has "Home Depot" or
"Walmart" as their neighbors, and that some of us can buy iodine and red phosphorus without getting raped by the "DEA". I don't particularly like the
USA, but that's not what I mean by my signature (and it's not a suitable topic of discussion for this forum).
Oh, fuck...
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
So, the USA is responsible for the rest of the world being incredibly overpopulated?
Be assured, that in twenty years or so, if things continue as they have been, 97.5% of the world's population will live outside the USA.
In much of the arid, starving, flea-bitten Mid-East, the population has doubled in the last 15 years.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Take the political talk to another forum. His signature was for chemistry material sourcing reasons, as he said. Not USA bashing. Politics and SciMad
dont mix very well. There are countless examples of this. Just check the locked threads...
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by zed  | So, the USA is responsible for the rest of the world being incredibly overpopulated?
Be assured, that in twenty years or so, if things continue as they have been, 97.5% of the world's population will live outside the USA.
In much of the arid, starving, flea-bitten Mid-East, the population has doubled in the last 15 years. |
Are you fucking blind? Did you even read my answer to triplepoint's post? If yes, what part of it didn't you understand?
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Weird!
This "So, the USA is responsible for the rest of the world being incredibly overpopulated?"
isn't just spurious: it's wrong.
The world's mean population density is about 14 people per square km. The US figure is about 34.
So the US is overpopulated compared to the rest of the world, rather than the other way round.
But, since nobody said that the US was responsible for anything anyway...
http://en.wikipedia.org/wiki/List_of_sovereign_states_and_de...
|
|
|