methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
Addition elimination mech question
hello everyone one,
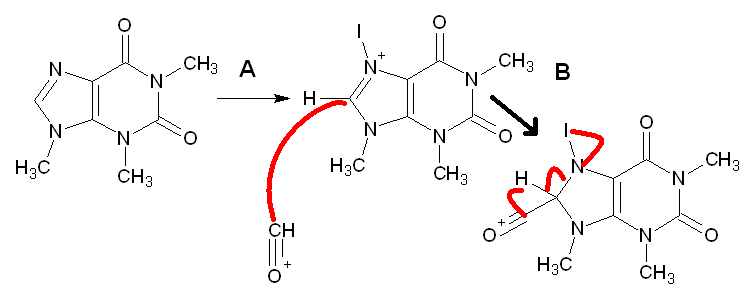
I have attached a proposed mechanism for the formylation of a purine.
I am generating the I^3 species in stichu to form the more favored N-iodo species which should activate the 8 position toward addition of a strong
nucleophile. my question is concerning the mech can someone tell me if I am going to the right methodology.
(note chem sketch wont allow me to make CO)
|
|
|
methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
correction: the second intermediate should not have a double bond in the imidazole ring.
rather showing electron migration to the nitrogen
[Edited on 6-3-2012 by methylene_beam]
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
make the picture smaller, add the correct arrows in chem draw. this is a very ugly mechanism!
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
I can't quite tell but it seems you are trying to take away electrons from an already positively charged oxygen, that isnt going to work.
|
|
|
methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ThatchemistKid  | | I can't quite tell but it seems you are trying to take away electrons from an already positively charged oxygen, that isnt going to work.
|
I am attempting to show the carbonyl carbon(from CO) picking up the imidazole proton allowing for the aldehyde functionality and the return of its
aromatic state by ejection of the I- on the nitrogen.
The black dot represents the carbonyl carbon.
1) positively charged oxygen attacks the carbonyl carbon.
2) the aromatic proton eliminates
3) elimination of the aromatic proton causes re formation of the aeromatic ring eliminating the N-iodo intermediate.
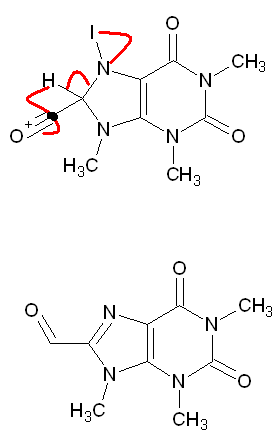
[Edited on 6-3-2012 by methylene_beam]
|
|
|
Nicodem
|
Thread Moved
7-3-2012 at 09:18 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm sorry to tell you that not much of what you depicted makes sense (to me). Despite your poor skills in using structure drawing software and
unawareness of the meaning of mechanistic arrows, I understood that what you want to describe is a hypothesis that I<sub>2</sub> could
catalyze the formylation of caffeine with carbon monoxide. You seem to believe that I<sub>2</sub> is inert to CO at the reaction
conditions (without giving a reference). Thus, the carbocation generated by the electrophilic attack of the iodine at the nitrogen would in your
(referenceless) opinion of all things react with the carbene (CO) rather than the iodide (admittedly this is reversible) or any other nucleophile
present in the reaction medium (possibly irreversibly). Such a reaction would then give an acylium cation which is quite thermodynamically uphill and
also very reactive. However, you seem to (referencelessly) believe that this species would not react with any of the plenty of nucleophiles present in
the medium before it would have the time to undergo a 1,2-hydride shift to give the formyl derivative of caffeine. Is this what you are trying to
describe and ask about? Well, I do not know if it works, but I have an irrelevant opinion about it.
PS: Please open referenceless threads only in the Beginnings section.
|
|
|
methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | I'm sorry to tell you that not much of what you depicted makes sense (to me). Despite your poor skills in using structure drawing software and
unawareness of the meaning of mechanistic arrows, I understood that what you want to describe is a hypothesis that I<sub>2</sub> could
catalyze the formylation of caffeine with carbon monoxide. You seem to believe that I<sub>2</sub> is inert to CO at the reaction
conditions (without giving a reference). Thus, the carbocation generated by the electrophilic attack of the iodine at the nitrogen would in your
(referenceless) opinion of all things react with the carbene (CO) rather than the iodide (admittedly this is reversible) or any other nucleophile
present in the reaction medium (possibly irreversibly). Such a reaction would then give an acylium cation which is quite thermodynamically uphill and
also very reactive. However, you seem to (referencelessly) believe that this species would not react with any of the plenty of nucleophiles present in
the medium before it would have the time to undergo a 1,2-hydride shift to give the formyl derivative of caffeine. Is this what you are trying to
describe and ask about? Well, I do not know if it works, but I have an irrelevant opinion about it.
PS: Please open referenceless threads only in the Beginnings section. |
I really don't understand where the condescending nature of this reply is coming from. If i wanted to talk to insecure scientists I would go to
academia with my questions.
anyway, I know people have been shitting on me about not using a program to draw these structures....I am sorry deal with it you know what i am trying
to depict. And why would i use anything but doubles headed arrows?
| Quote: |
You seem to believe that I<sub>2</sub> is inert to CO at the reaction conditions (without giving a reference). Thus, the carbocation
generated by the electrophilic attack of the iodine at the nitrogen would in your (referenceless) opinion of all things react with the carbene (CO)
rather than the iodide (admittedly this is reversible) or any other nucleophile present in the reaction medium (possibly irreversibly).
|
I am gong off the methodology that you posted in about one of my threads. and I said in that thread that the I3 species didn't react and returned
starting material. I am going off your methodology presented and adding a more reactive nucleophile.
remember when you posted this??
http://www.sciencemadness.org/talk/files.php?pid=236202&...
You told me(in the other post) to use an inert solvent. dont you think thats what i am doing???
| Quote: |
However, you seem to (referencelessly) believe that this species would not react with any of the plenty of nucleophiles present in the medium before
it would have the time to undergo a 1,2-hydride shift to give the formyl derivative of caffeine.
|
you keep mentioning that i don't have references.... of course i don't thats why i am asking the question lol. I am hoping someone with mechanistic
knowledge can simply be like.
"no that wont work cause of ABC"
or
"maybe give it a try tell us what happens"
if iodo-phosgene in generated in situ it would only make the carbonyl carbon more reactive. maybe forming the polymerized product.
edit: why was this moved to "beginings" this subject matter is farm from that. now people arent going to read this, thanks........
alls i wanted was a yes or no. Its a shame when egos get involved
[Edited on 7-3-2012 by methylene_beam]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by methylene_beam  | | anyway, I know people have been shitting on me about not using a program to draw these structures....I am sorry deal with it you know what i am trying
to depict. And why would i use anything but doubles headed arrows? |
It's obvious I'm talking to my self here, given that evidently you did not even bother to carefully read my reply above, but I will nevertheless try
again. Like I said, I tentativelly understood your hypothesis even though you are unable to depict it or use mechanistic arrows. The problem you face
has nothing to do with your drawing skills or rules in regard to arrow use. Your major problem is your disregard of the scientific method. You propose
a mechanism for a reaction that does not even exist. What is worse, your hypothesis is not based on a single reference! That is not even a hypothesis
any more. Regardeless of what you think, I'm actually trying my best to help you. The problem is that you are unable to accept any help for which you
would have to pay with discipline, work and devotion. You want something, but you don't want to pay for it. So, who is having an ego problem here?
| Quote: | | I am gong off the methodology that you posted in about one of my threads. and I said in that thread that the I3 species didn't react and returned
starting material. I am going off your methodology presented and adding a more reactive nucleophile. |
Like it was already obvious to me at the time I wrote that reply, you misinterpreted what I wrote. Besides, since when is carbon monoxide a more
reactive nucleophile than iodide, water and other things that you would want to have in the reaction mixture? Do you have a reference for that?
| Quote: | | You told me(in the other post) to use an inert solvent. dont you think thats what i am doing??? |
I said that it is advisable to use an inert nonucleophilic solvent if you want a higher chance of success. But on the other hand, you don't propose
any reaction conditions for the non-existing reaction you propose in this thread. We are only left to guess you are talking about aqueous conditions
due to your mention of the triiodide anion. Water is not a nonnucleophilic solvent.
| Quote: | | you keep mentioning that i don't have references.... of course i don't thats why i am asking the question lol. I am hoping someone with mechanistic
knowledge can simply be like. |
Now, what was that about? You want a scientific comment in regard to a poorly presented referenceless hypothesis of an imaginary reaction and you get
pissed because you didn't understood the reply? What's worse, you don't even find all this absurd.
| Quote: | "no that wont work cause of ABC"
or
"maybe give it a try tell us what happens" |
Exactly the kind of reply that I gave you already. Since you are obviously not going to do any experimental research, you are forced to try and
understand what that ABC I told you means. Obviously, this is not going to happen unless you do some literature search and reading. Coincidentally,
that is exactly my final goal.
| Quote: | | edit: why was this moved to "beginings" this subject matter is farm from that. now people arent going to read this, thanks........
|
I moved the thread here so that you would be given a chance to learn step by step, rather than to just read replies without actually reading
them.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
I am done with this place. This forum is for drug cooks and old guys with a chip on their shoulder. I have asked simple questions about things I am
curious about and have been meet with hostility the entire time.
I have read other posts you have been in nicodem and your an asshole bro. see ya
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Without heads on the curly arrows, there is no way to show which way the electron is flowing. Nicodem does have a point there.
|
|
|