| Pages:
1
2 |
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
NH3 (aq) or NH4OH
So right now, I am trying to figure out weather aqueous ammonia is just an auqeous solution of it, like it should be, or weather it is actually
ammonium hydroxide ( which doesn't make much sense). I read in an ancient chem text, that ammonia (household) is really a week solution of NH4OH. I
think that that information may be outdated. So aqueous household ammonia is a base, so that means that it must contain an -OH ion. Correct? (I
know...Lewis acid blah blah blah...let's
keep it basic  ) But isn't gaseous ammonia NH3 a base? ) But isn't gaseous ammonia NH3 a base?
Chemically yours
Mirage
[Edited on 5-2-2012 by Mirage]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
When ammonia dissolves in water it forms ammonium hydroxide, NH4OH. As a gas, ammonia has the formula NH3.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Some partial ionisation occurs so it is both solution and base . . .
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
Ammonia isn't ionic. Correct? The equation would look a bit like an acid dissasociating...correct? What would it be...
NH3 + H2O <---> NH4+ + OH-
What side would be more favored, I'm expecting the aqueous ammonia to be more favored.
Thanks
Mirage
[Edited on 6-2-2012 by Mirage]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Ammonia in solution is a weak base, and mostly present in undissociated form. Equilibrium constant for reaction NH3 + H2O → NH4(+) +
OH(− is 1,8×10−5 at normal conditions, meaning equilibrium is shifted
to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous
ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction
of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase. is 1,8×10−5 at normal conditions, meaning equilibrium is shifted
to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous
ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction
of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase.
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
I see. well thank you everyone for your help.
| Quote: | | Ammonia in solution is a weak base, and mostly present in undissociated form. Equilibrium constant for reaction NH3 + H2O → NH4(+) + OH(−
is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved
NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for
solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can
not contain ions so term base can't be used for ammonia in gas phase. |
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not really, no.
Most of the ammonia is just dissolved.
Some proton transfer happens so there are a few (about 10 in a million) NH4+ ions, but these are not associated with any particular OH- ions so
there's no real NH4OH present.
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like
trace compounds.
Mirage
Quote: Originally posted by unionised  |
Not really, no.
Most of the ammonia is just dissolved.
Some proton transfer happens so there are a few (about 10 in a million) NH4+ ions, but these are not associated with any particular OH- ions so
there's no real NH4OH present. |
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mirage  | | Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like
trace compounds. |
What he is saying that there is no such thing as NH4OH. The pKa of the ammonium cation in water is about 8.9, so there is a very, very unfavorable
equilibrium for its formation. Yet, that little that forms can only exist as solvated NH<sub>4</sub><sup>+</sup> cations and
not as ammonium hydroxide which is an unknown compound anyway. The name "ammonium hydroxide" is an old trivial name that was used in some countries
for aqueous solutions of ammonia - it is not a name for a compound corresponding to the actual empirical formula of NH4OH. I hope this is clear
enough.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
Crystal clear! Finally! Thanks for everyone's help!
Mirage
Quote: Originally posted by Nicodem  | Quote: Originally posted by Mirage  | | Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like
trace compounds. |
What he is saying that there is no such thing as NH4OH. The pKa of the ammonium cation in water is about 8.9, so there is a very, very unfavorable
equilibrium for its formation. Yet, that little that forms can only exist as solvated NH<sub>4</sub><sup>+</sup> cations and
not as ammonium hydroxide which is an unknown compound anyway. The name "ammonium hydroxide" is an old trivial name that was used in some countries
for aqueous solutions of ammonia - it is not a name for a compound corresponding to the actual empirical formula of NH4OH. I hope this is clear
enough. |
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Wow I really thought NH4OH was the main constituent of NH3+H2O...
Interesting.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Was told by one of my chemistry teachers that ammonium hydroxide does not exist, and is just a simplification (as mentioned by Nicodem).
Ammonium hydroxide does not exist (Read the letter/abstract)
Ammonia and "ammonium hydroxide" (I have no acces, this is just the article referred to in the above letter/abstract)
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Why the hell would NH4+ form but not OH-?! Where is it getting its hydrogen? Other ammonia? Like NH4+ NH2-?
BOLD
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
spam reported.
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
Entropy51, so many of your posts lately have been made up of snide, rude, and insulting remarks toward other members. I don't know what your problem
is, but a little civility isn't too much to ask for. It's one thing to disagree with someone, but its a whole other thing to simply post for the sake
of being a complete and utter asshole.
[Edited on 2/7/2012 by UKnowNotWatUDo]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@entropy51:  Stop digging up old and unrelated quotes! GreenD only wrote about
the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you Stop digging up old and unrelated quotes! GreenD only wrote about
the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you 
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What unionised says is that each NH4(+) ion is not associated to a particular OH(-) ion. You cannot say
THIS NH4(+) ion is bound to/associated with THAT OH(-) ion. Of course, for each NH4(+) there also is one OH(-). There will be no NH2(-) in solution.
Just look at it as when you dissolve NaCl in water. In the solid, each Na(+) ion is surrounded by a few Cl(-) ions, and these ions are fixed at their
position and there is an association between ions. If you could label each ion (e.g. with a name), then you could say that ion with name A is next to
ion with name B and this remains true as long as the solid exists. In solution, however, the Na(+)/Cl(-) lattice breaks up and the ions can move
through the liquid independent of each other. Ion A can for instance move to one end of the beaker, while ion B moves to the other end of the beaker.
You only can say that for each Na(+) ion there is one Cl(-) in solution, but you cannot associate couples of ions to each other.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Thanks for the response on my behalf. The connections he is making in his post are far from reality.
It was not my choice to label a NH3 aq solution as sodium hydroxide.
So what would the pH of a 30-35% NH3 (aq) solution be?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
From this data here, I would guess at just over 12
http://www.airgasspecialtyproducts.com/Libraries/Aqua_Ammoni...
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, pH will be around 12. Normal household ammonia has a pH around 11. This means that the concentration of OH(-) is really low, even in concentrated
solutions, in the order of 0.001 M to 0.01 M. The concentration of NH3 is around 3 M for normal household ammonia and the concentrated stuff has
around 15 M, or even higher. This nicely shows how weak a base ammonia is.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
I think I understand. We are not debating that there is a large portion of NH4(+) molecules in solution, right? (.01-.0001M)
We are debating that the molecule NH4OH exists.
NOW this makes sense. I thought everyone was trying to tell me that NH4+ is not being formed. but it just the complex NH4OH ! Right? but it just the complex NH4OH ! Right?
This would be the same argument that H4O2 doesn't exist, right - H3O+/OH- molecule?
[Edited on 7-2-2012 by GreenD]
|
|
|
Vikascoder
Hazard to Others
  
Posts: 309
Registered: 28-1-2012
Member Is Offline
Mood: No Mood
|
|
Ph refers to power of hydronium ion even the diluted naoh have ph of 14 but its concentration is low so why is the difference of 12 to 11 in ammonia
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by Vikascoder  | | Ph refers to power of hydronium ion even the diluted naoh have ph of 14 but its concentration is low so why is the difference of 12 to 11 in ammonia
|
Sodium hydroxide is highly dissociated into solvated sodium and hydroxide ions in water so strong solutions are all around pH14 and even quite dilute
solutions are still strong bases, it is only very dilute soltions that are substantially less than that.
Ammonia solutions are quite the opposite, dilute solutions are mainly dissolved ammonia with a small proportion of ammonium and hydroxide ions,
concentrated ammonia solutions contain much more dissolved ammonia but not much more ammonium and hydroxide ions.
I have had a look at this Wikipedia article and I think it is a good explanation;
http://en.wikipedia.org/wiki/PH
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
It looks like the hydrogen bond in un-dissociated NH<sub>4</sub>OH is longer than usual because of the weaker coulombic attraction by the
nitrogen.
At the MP2/6-311G(3df2p) theory+basis level, the model also doesn't permit a straight line H-bond between the N and O, unlike in water.
Here is a model of the HOMO-2 molecular orbitals that show the essential connection and geometry.
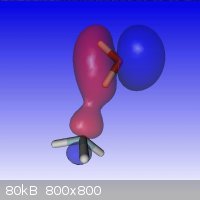
|
|
|
Mirage
Harmless

Posts: 49
Registered: 26-1-2012
Location: Canada
Member Is Offline
Mood: Superheated
|
|
You lost me at "Columbia attraction"  ... ...
Quote: Originally posted by arsphenamine  | It looks like the hydrogen bond in un-dissociated NH<sub>4</sub>OH is longer than usual because of the weaker coulombic attraction by the
nitrogen.
At the MP2/6-311G(3df2p) theory+basis level, the model also doesn't permit a straight line H-bond between the N and O, unlike in water.
Here is a model of the HOMO-2 molecular orbitals that show the essential connection and geometry.
|
|
|
|
| Pages:
1
2 |