Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Pathways for a beginner
Over the past couple years I've been gathering a variety of OTC
chemicals and a small amount of glassware to teach my 9 and
11-year-old boys a bit about chemistry. I'm a former engineer,
now studying to be a sculptor so I haven't had a lot of time to
devote to the project; progress has been quite slow. So far
we've made soap, CO2, voltaic cells -- the usual ultra-simple
home science stuff. As might be expected, this stuff has been
more yawn provoking than inspirational to them, so I'm looking
for more interesting stuff to do with them.
I took inorganic chem in university back in the early 1980s, just
the one class required to get my engineering degree. It wasn't
because I wasn't interested but because it was a mega-lecture
class with 300 other students. I didn't take the corresponding
lab section (scheduling conflict), and I've always regretted that
because I was pretty good in the lab in high school chem. I want
to hone my lab skills and finally learn something about organic
chemistry. Right now I reckon I'm still trying to learn enough to
just find out what it is I need to learn just to ask intelligent questions!
So far I know I'm interested in photographic processes, so making
various silver salts would be in the cards (after making nitric
acid & silver nitrate), aromatics look fun, particularly making
Bakelite, oil of wintergreen, & aniline pigments, and maybe
make photo-reactive polymers (I have this dream of building
a rapid prototyper some day...).
I'm looking for suggestions for simple reactions/procedures that
include one or more of the following attributes:
Have way more pizzaz than vinegar & baking soda volcanoes;
Involve synthesising/purifying as many of the reagents needed as
is practical at home, either as byproduct of previous experiments
or as experiments in their own right;
Demonstrate unambiguously principles of chemistry or good lab
technique.
To the above I must add the constraint that it must be low-cost,
on the order of a dollar or two a day for equipment and supplies.
This is a big issue because I was recently quoted $86AUD for
250g of phenol crystals, $33AUD for 500mL of formaldehyde, and
$32AUD for 100mL of phenolphthalein soln. I have no idea if
these are reasonable prices, but know if we make Bakelite,
this would blow our budget for three months for just one experiment!
Thanks for any help y'all might be able to give.
-Bobby
[Edited on 13-9-2010 by Gearhead_Shem_Tov]
[Edited on 13-9-2010 by Gearhead_Shem_Tov]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Have you seen the Golden Book of Chemistry Experiments, available online?
It might not meet all your criteria, but it has a lot of interesting experiments that can be done with readily available (at least in the U.S.)
chemicals and it was designed for the age group of your sons, more or less.
|
|
|
Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by entropy51  | Have you seen the Golden Book of Chemistry Experiments, available online?
It might not meet all your criteria, but it has a lot of interesting experiments that can be done with readily available (at least in the U.S.)
chemicals and it was designed for the age group of your sons, more or less. |
I do have the Golden Book of Chemistry ebook. I also have a modest
collection of hardcopy books on amateur chemistry:
Dunn's Caveman Chemistry
Thompson's The Illustrated Guide to Home Chemistry Experiments
Wailes' The Home Chemist
Yates' How to Make and Use a Small Chemical Laboratory
Holden & Singer's Crystals and Crystal Growing
I also have a pile of industrial process, formulary, and receipt-
type books (mostly from Lindsay Books), as well as dozens of ebooks.
-Bobby
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Gearhead I know what you mean about producing yawns from an audience which has as yet no grounding in chemistry fundamentals. I guess what they want
to see is quick color changes, fizzing, burning, stinking, small explosions, and other quick and impressive audio/visual demos. These are fun, even
for old farts like me, but how much chemistry is really relayed, or how much interest in chemistry is really inspired? I guess you have to start
somewhere, and get their attention first. Slip a little theory in while they aren't looking.
I found those prices you quoted to be completely ridiculous, assuming 1$AUS roughly equals 1$US. Search around on the secondary chemical market,
hobby, and OTC markets. That's where I get 95% of my chemicals. These sources are very cheap compared to the likes of Fisher, VWR, Aldrich, etc.
I have always found colorimetric titrations very satisfying. You get a nice color change and learn how very quantitative chemistry can be. Crystal
growing is slow but eventually can be impressive and rewarding. Precipitations are also very interesting visually and present a good chance to slip
in some theory. Electrolysis of water is another good demo.
[Edited on 14-9-2010 by Magpie]
[Edited on 14-9-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Some of the old books of chemistry lecture demonstrations, such as this one have some interesting, and even some spectacular, demonstration experiments.
|
|
|
Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | Gearhead I know what you mean about producing yawns from an audience which has as yet no grounding in chemistry fundamentals. I guess what they want
to see is quick color changes, fizzing, burning, stinking, small explosions, and other quick and impressive audio/visual demos.
|
Yeah, I reckon I need to come up with a balance of what I'm interested in versus what will catch their imaginations.
| Quote: |
These are fun, even for old farts like me, but how much chemistry is really relayed, or how much interest in chemistry is really inspired? I guess
you have to start somewhere, and get their attention first. Slip a little theory in while they aren't looking.
|
Maybe I need to do the "serious" stuff -- titrations, purifying crude chemicals into reagents, etc -- on my own. And punctuate
that with the Mr. Wizard schtick for them.
| Quote: | I found those prices you quoted to be completely ridiculous, assuming 1$AUS roughly equals 1$US. Search around on the secondary chemical market,
hobby, and OTC markets. That's where I get 95% of my chemicals. These sources are very cheap compared to the likes of Fisher, VWR, Aldrich, etc.
|
I have gotten all my chemicals OTC to date. I had a particularly good OTC day last week when, in one day, I got a litre each
of 100% toluene and 100% methanol, and half a litre of 50% H2O2, the lot for under $30AU. I could have gotten five times
as much of each, no raised eyebrows, no paperwork hassles.
The peroxide came from a hydroponics shop, of which Adelaide has a bustling surplus. I've heard some hydroponics shops
sell nitric as ph down, but I've never seen any yet.
It's frustrating what I can't find. I bought a cheap 25 kilo bag of kno3 two years ago, but I can't find cheap
formaldehyde. And none of the local photo chemical shops even carry GAA anymore!
| Quote: | I have always found colorimetric titrations very satisfying. ... Crystal growing is slow but eventually can be impressive and rewarding.
Precipitations are also very interesting visually and present a good chance to slip in some theory. ...
|
All good points. I've got a Rochelle salt soln. sitting out now waiting for crystals to form. It's been five days, but still no sign of crystals at
all.
-Bobby
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Bobby those are some good OTC finds, especially the 50% H2O2. I can buy 35% OTC but it's still expensive. Check the pet stores for formalin,
including the mail order places.
Here's a thread with my pictures of Rochelle salt, which grew quite quickly:
http://www.sciencemadness.org/talk/viewthread.php?tid=6376#p...
In another thread I post the recipe:
"Weighed out 39g Cream of Tartar and added to 49 mL of water. Placed slurry in 250mL beaker in water bath using Corelle bowl on stirrer-hotplate.
Heated until bath water at simmer. Added washing soda (Na2CO3) carefully with spatula until no more bubbles formed. Filtered hot solution into
evaporating dish. ~2 hours later 7 large crystals of Rochelle salt had formed."
Good luck.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Getting to phenols
After we master the basics of purifying chems, I want to get into
polymer chemistry, phenolics in particular. I've spent a lot of time
daydreaming about various unorthodox feedstocks for phenols, and
I happened to look at the structure for d-Limonene, and for a
moment I got excited. Wow, I can make plastics from lemon peels
instead of toluene, I thought -- until I looked closer:
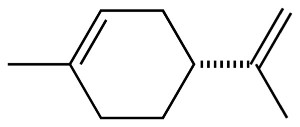
Nope, that's not an aromatic, that's a cyclic terpene. So, buying a
barrel of d-Limonene solvent isn't going to work as precursor for
Bakelite (though it would make my lab smell lemony fresh...).
Seriously, though, what would be a more benign precursor for
phenol, benzene, etc. than toluene? Or is toluene the "best of all
possible worlds" already? Am I even asking the right question?
-Bobby
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
Well I can tell you toluene far exceeds benzene in benign-ness
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
kids love bangs! have you thought about nitrogen triiodide? easy to make and it used to be done in middle school classes as a demonstration for the
same reasons you mention(ie the BORING lectures). also instead of just mixing ammonia solution with iodine you can really demonstrate the chemistry
involved by making your own iodine from potassium iodide or sodium iodide, water, HCl, and H2O2, then make your own ammonia solution by bubbling the
gas from mixing ammonium nitrate with sodium hydroxide through water. pour the ammonia solution over the iodine and allow to sit for five minutes then
filter. leave the filtrate to sit and DO NOT DISTURB. after it dries touch it with a stick and bang! this is potentially dangerous so do not make more
than a quarter gram at a time. it is very sensitive to touch, breeze, even looking at it funny so once you filter it, set it where you want to
activate it and DO NOT DISTURB!!! i performed this same experiment for my own kids and they loved it. hope this post doesn't get me in trouble!
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also show my kids some more spectacular experiments. A very nice one is bubbling acetylene gas into chlorine gas:
http://woelen.homescience.net/science/chem/exps/cl2_c2h2/ind...
Chlorine is easy to make (pool chlorinator + dilute hydrochloric acid) and acetylene can be made from calcium carbide, which also can be purchased
easily.
Actually, I have a special section in my website with 'kewl' experiments. Although this is not the kind of chemistry which first comes to mind when I
think of chemistry, it certainly helps raising interest with young kids.
http://woelen.homescience.net/science/chem/exps/index_fire.h...
Experiments, aimed at kids of 12 years:
http://woelen.homescience.net/science/chem/exps/child/
|
|
|
HydroCarbon
Hazard to Self
 
Posts: 77
Registered: 7-7-2008
Location: Anytown, USA
Member Is Offline
Mood: No Mood
|
|
Dealing with phenol, formaldehyde, and benzene seem a bit toxic for home experiments with the kids. Especially if you don't have too much chemistry
experience.
Some of these have been mentioned before but some good ones are: steam distillation for essential oils, water electrolysis, homemade gunpowder
(experiment with different formulations), making soap, precipitation reactions... etc.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
props to you for that! anything to spark interest in the next generation of scientists! you never know...one of them may be the one to cure cancer (if
they can get past the pharm companies. you know...the money is in the treatment not the cure). but if we don't encourage experimentation in the young,
science will be on a downward spiral till it crashes altogether.
|
|
|