JimmySuperStar
Harmless

Posts: 1
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
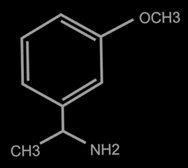
Beta-Methoxy Phenylethlyamine
What do you think,what is the easyest way to make Beta-Methoxy Phenylethlyamine ?
it is one of 3 supstances in sport supplemnt "DREN"
|
|
|
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
The correct name of this substance is 3-methoxy-alpha-phenylethylamine or 1-(3-methoxyphenyl)-ethylamine.
You could start with 3-methoxyacetophenone and ammonium formate/formic acid (Leuckardt-Wallach-reaction).
Precursor acetophenone:
1. 3-nitroacetophenone (nitration of acetophenone? not sure) ---> reduction to 3-aminoacetophenone
2. 3-aminoacetophenone ---> diazotize, heat, reaction to 3-hydroxyacetophenone
3. 3-hydroxyacetophenone + methylation agent like dimethyl sulfate ---> 3-methoxyacetophenone
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Two points:
Nitration of acetophenone is a toughie, seeing as its less reactive than benzene itself. Not sure what kind of conditions you'd need, but I'd guess
reflux and higher conc. nitric acid than usual in mixed acids, if not the use of oleum.
The methoxy group may be furnished directly by diazotising and heating in methanol. Needless to say, any water around would compete. But it saves
another step and the use of a nasty reagent like dimethyl sulfate.
A more creative (albeit difficult for the amateur to pull off) is to react anisole to for them chromium tricarbonyl complex. This may then undergo
nucleophilic attack with the nitronate of nitroethane, although this should be checked as I cannot remember the regiochemistry of such a reaction. The
chromium complex is then reduced with iodine, and the nitro group can finally be converted to the amine using the "textbook" Sn/HCl reduction.
|
|
|
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
Yes, another good possibility. What are typical average yields of this kind of transformation?
Do you expect the yield of this one-step-method exceeds the two-step-process?
In this case your process would be superior of course.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Well it will only be superior if the yeilds are appropriate. This is just something I cobbled together in a couple of seconds. I see no reason why it
shouldnt work as effeciently as the phenol synthesis though. And I have seen such a reaction referenced somewhere, so I know it definately works (its
beyond speculation).
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
The meta nitration of acetophenone should take place in a mixture of concentrated sulfuric and nitric acids at 0ºC. Should take maybe 20minutes or
so. Really don't need to reflux to get mono-nitration.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
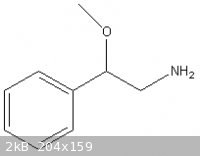
JimmySuperStar took name of that molecule from supplement "DREN" list of ingredients, so I suppose he made mistake in drawing incorrect structure, not
in putting incorrect name above it.
So what he asks us to do, is to search for synthesis of Beta-Methoxy Phenylethlyamine.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | Nitration of acetophenone is a toughie, seeing as its less reactive than benzene itself. Not sure what kind of conditions you'd need, but I'd guess
reflux and higher conc. nitric acid than usual in mixed acids, if not the use of oleum. |
http://www.orgsynth.org/orgsyn/prep.asp?prep=cv2p0434
| Quote: | | The methoxy group may be furnished directly by diazotising and heating in methanol. Needless to say, any water around would compete. But it saves
another step and the use of a nasty reagent like dimethyl sulfate. |
No, it is not possible. Diazonium salts preferentially decompose forming a phenyl radical whereever possible (Sandmeyer reaction), often even without
copper catalysis, rather than the phenyl carbocation trough which the reactions with F- (BF4(-)) and O-nucleophiles (H2O) proceed. That is why their
decomposition in alcohols (methanol included) gives almost exclusively ArH from ArN2(+). Traces of anisols might form in methanol (via carbocation),
but the main pathway is the thermodynamically more favourable dediazotation trough alpha-hydrogen abstraction from alcohols (via phenyl radical).
There was a paper about this posted time ago in another thread somewhere where this possibility was brought up.
| Quote: | | So what he asks us to do, is to search for synthesis of Beta-Methoxy Phenylethlyamine. |
The synthesis of 2-methoxy-2-phenylethylamine from styrene in two steps is described at the Hyperlab forum. Besides there are plenty of other
syntheses described in the literature, but given that it is not even certain this is the compound he asks about, I will certainly not waste time
searching for examples.
I'm moving this thread to Beginnings as it is not even clear what compound is all this about. Besides the original poster was even too lazy to add a
single reference to support his claims and resolve the confusion about what he is asking at all.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
7-11-2009 at 02:33 |
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
Oh, I think you are right, Satan. An important detail, easy to overlook in the light of Jimmy´s formula which appeared so likely considering the fact
that the names of substances in the field of medicine are often not scientifically correct  ... ...
But now the adapted synthesis:
1. acetophenone + halogene (Cl2 or Br2) ---> 2-haloacetophenone
2. 2-haloacetophenone + NaBH4 ---> 2-halo-1-phenylethanol (avoid epoxide formation!)
3. 2-halo-1-phenylethanol + dibenzylamine ---> 2-dibenzylamino-1-phenylethanol
4. 2-dibenzylamino-1-phenylethanol + SOCl2 ---> 2-dibenzylamino-1-phenylethylchloride
5. 2-dibenzylamino-1-phenylethylchloride + NaOCH3 ---> dibenzylprotected final product
6. cat. hydrogenation ---> final product
Very laborious, but works definitely.
Edit: I didn´t see the very easy synthesis posted by Nicodem.
[Edited on 7-11-2009 by grind]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nicodem: You are right about using alcohols for diazotonium decomposition yields arenes rather than phenyl alkyl ethers. I didnt realise the yields of
the ether was so low though, for example phenyldiazonium tetrafluoroboarate with methanol gives benzene in 98% yeild, the remainder being anisole.
However, on searching to clarify this, I found an interesting paper that claims the ether in 51%, quite remarkably. The downside is that the reagent
is not the simple alcohol, but the alkoxytrimethylsilane. Here is the paper DOI: 10.1055/s-1991-26419
|
|
|