jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
At what point does Sulfuric Acid reach its lowest Ph?
Sulfuric is diprotic so it has 2 hydrogens per each mole - so for each mole (98 grams) 2 moles of water are needed 18 + 18 grams
98+18+18 = 134
98/134 = roughly 78%
so the highest concentration at which sulfuric is still completely dissociated is a 78% solution - but I also have read that sulfuric can dissociate
itself? does sulfuric stay completely dissociated above this concentration?
I know I am probably missing something..
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
If I remember right, even HCl does not fully dissociate at a concentration of 0,1M. And HCl is a stronger acid than sulfuric acid!
Or was it something with ion activity? Anyway, a 0,1M solution does not have pH 1,00, but somewhere above that. We did an experiment at uni today, and
we calculated with a formula, wich I cannot recall at the moment for some reason  , what the pH should be of a 0,1M, 0,01M, 0,001M and 0,0001M solution of KCl in 0,001M HCl. We also calculated the pH of a 0,001M HCl solution. It
was around 3,15. And this was right, when we tested it! The influence of KCl is on the ion activity, but that is offtopic. , what the pH should be of a 0,1M, 0,01M, 0,001M and 0,0001M solution of KCl in 0,001M HCl. We also calculated the pH of a 0,001M HCl solution. It
was around 3,15. And this was right, when we tested it! The influence of KCl is on the ion activity, but that is offtopic.
Anyway I can assure you that at 78% sulfuric acid does not lose all it's proton's, as described above HCl does not even do this at 0,1M
concentrations! There will be almost NO sulfate, only bisulfate and free H2SO4. Sulfuric won't even lose all of it's first protons at 78% !
[Edited on 23-1-2009 by Jor]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Jor is right, even strong acids have no 100% ionization.
Besides that, at very high concentrations, the concept of pH is not valid anymore. The concept of pH is based on the assumption that the concentration
of water is a constant (55 mol/l) and this is a valid approximation for the pH-scale from 0 to 14. Below 0, the concentration of dissolved acid
becomes so high that the concentration of water becomes noticeably lower and the same is true above pH=14 for strong bases. For rough pH calculatons,
even a pH of -0.3 or so can be used or a pH of +14.3 but the approximation becomes worse rapidly at even lower values (and higher values for bases).
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
Ok - so acids never fully dissociate - then let me rephrase - at what concentration is sulfuric at its most dissociated point? Ph doesn't apply at
high concentrations? well I guess that could be right - it would be rough to measure after a certain point - I was guessing that 2 moles of water
would be the least amount needed to provide the needed hydrogen to form the hydronium radicals but since full dissociation doesn't happen maybe less
water is needed..
|
|
|
HydroCarbon
Hazard to Self
 
Posts: 77
Registered: 7-7-2008
Location: Anytown, USA
Member Is Offline
Mood: No Mood
|
|
I would think it would depend on whether you want the highest percentage of dissociation of total H2SO4 molecules, or just highest amount of H3O+
possible.
For the highest percentage of dissociated H2SO4 molecules you would probably want an excess of water and a lesser amount of acid. The reasoning
being, if there is more water than acid then there are more chances for the protons to come off the molecule.
For the strongest possible acid solution, however, dissolving the acid to saturation would most likely give you the most total protons in solution per
volume of water.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
@Jor: Wasn't the rule for calling an acid stronger than another, that it can displace the other from it's salts ?
Thereby H2SO4 would be the stronger acid, not HCl .
|
|
|
kmno4
International Hazard
    
Posts: 1502
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
From conductivity measurements in acetic acid, dissociation constants for HNO3, HCl, H2SO4, HBr, HClO4 are like:
1:9:30:160:400.
But it is commonly known that HBr or HClO4 can be prepared from KBr or KClO4 and H2SO4 (even 97%); KClO4 does not react with conc. HCl
For concentrated solutions, pH "turns" to H<sub>0</sub>.
http://en.wikipedia.org/wiki/Hammett_acidity_function.
BTW. A picture of H<sub>0</sub> = f(c) for some acids in water (from Kortüm's book). There is no local minimum on
any of these curves.
[Edited on 23-2-2009 by kmno4]
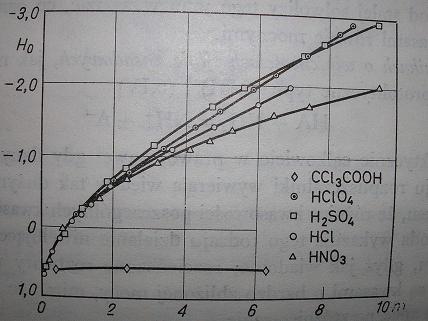
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I should think that sulfuric acid is most highly dissociated at infinite dilution. No? Let's look at what we know about pKa. When pKa = pH the acid
is 50% protonated and 50% deprotonated (though this is more complicated since it's it diprotic). At infinite dilution, the pH is still far above the
pKa (since pH is dependend on hydronium concentration), and hence the predominant form is the deprotonated species. The pKa of H2SO4 in H20 is
-3.0, so if we add HBr (pKa=-9.0) to the point that our pH is below -3.0 (theoretically speaking) H2SO4 will exist predominantly as the protonated
species. Since pH is a logarithmic scale, for each 1 pH unit above the pKa of the acid, there exists 10x greater amount of deprotonated species.
@Woelen - pH is still valid in DMSO, is it not? Albeit pKa's change as well.
[Edited on 23-2-2009 by Arrhenius]
|
|
|
len2
Harmless

Posts: 32
Registered: 13-9-2008
Member Is Offline
Mood: No Mood
|
|
That dont work.
If pK1 for H2SO4 = -3 then
[H2SO4] = ([H]/10^3) [HSO4]
without added HBr, [H] ~ q where q is conc. total H2SO4 added.
Lets say we add total HBr conc = c, and its totally dissociated
[H2SO4] = ( ([HSO4]+c)/10^3 ) [HSO4]
we cant hope to get c much above 10 and so
[H2SO4] ~ [HSO4]/100 at best
and you cant force H2SO4 to be substantially protonated with any acid.
or: solve (q-y) = ( (y+c)/10^3 ) y for same result
A different issue is that pK1 ~ -3 assumes a weak solution in water, where [H] assumes [H3O] and other species. With decreasing water content this
'constant' drops as H2SO4 is a worse proton acceptor then water and the meaning of the whole concept of dissociation becomes hazy - thats why the
original question is ill defined, you need an operational definition of pH.
[Edited on 25-2-2009 by len2]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Hm... I'm rather confused by your calculations. Also, in dilute solution, the assumption that [H2SO4] = [H3O]+ is not valid. Please do note that
HBr protonating H2SO4 is a theoretical exercise.
So, for H2SO4 pKa(1) = -3, and pKa(2) = 1.96 . Do note that the HSO4(-) is a weak acid. So in this sense, the amount exising as SO4(2-) is
constrained by pKa(2).
I'm looking at my general chemistry book here, and it says: "Over the concentration rance shown [0.0020-0.010M], HCl, a strong acid, is essentially
100% ionized."(Petrucci, 8th Ed., pg.683). Now, it also shows an ionization curve for a weak acid, acetic acid, which is not unlike HSO4(-) except
weaker. "The percent ionization of H3CCOOH, a weak acid, increases from 4% in 0.010M to essentially 100% when the solution is extremely dilute. Note
that in extremely dilute solutions, the two acids would have virtually the same pH." (pg.683)
So let's do a calculation with H2SO4: Let's try 10^-8M, I'd say that's pretty dilute, so our definition of pH holds.
Here are the two contributing reactions:
1. H20 + H20 <---> H3O(+) + OH(-)
2. H2SO4 + H2O ----> H3O(+) + HSO4
& pKa(1)=-3 so Ka(1) = 10^3
Let's use an ICE table to figure this out
2. H2SO4 + H2O ----> H3O(+) + HSO4
initial conc. 10e-8M - - -
change -xM - +xM +xM
final (10e-8 - x)M - xM xM
so: Ka(1) = [H3O(+)][HSO4(-)]/[H2SO4] = x*x/(10e-8 - x) = 10^3... x = -1000 HUHH??? What did we just learn... Using this sort of calculation, pKa
cannot be <0, but it is for H2SO4. H2SO4 is essentially completely dissociated to HSO4(-).
So what is the pH of this solution? (I'll save you the calc., it includes the autodissociation of water) pH=6.98. [H3O(+)] = 1.05e-7M.
Let's look at the next weak acid-base reaction. NOTE: the equilibrium of this reaction is directly related to the concentration of H3O(+) from
reaction 2 if x is not significantly less than x!!
pKa(2) = 1.96, so Ka = 1.096x10e-2
3. HSO4(-) + H2O ----> H3O(+) + SO4(2-)
initial conc. 10e-8M - 10e-8M -
change -xM - +xM +xM
final (10e-8 - x)M - (10e-8 + x)M xM
Ka(2) = [H3O(+)][SO4(2-)]/[HSO4(-)] = [(10e-8 + x)*x]/[(10e-8 - x)] = 1.096x10e-2
so x = 9.99x10^-9M, and the total concentration of H30(+) is 1.15x10^-7M, and the resulting pH is 6.94.
As we increase the initial concentration of H2SO4, the equilibrium of reaction 3 lies further to the left. Hence, the more dilute the solution of the
acid, the more dissociated it is.

|
|
|
len2
Harmless

Posts: 32
Registered: 13-9-2008
Member Is Offline
Mood: No Mood
|
|
Dilute solutions to which you are referring now, where you have to take account of water equilibrium, are not the negative pH solutions, to which you
were referringin the previous post
| Quote: | Originally posted by Arrhenius
The pKa of H2SO4 in H20 is -3.0, so if we add HBr (pKa=-9.0) to the point that our pH is below -3.0 (theoretically speaking) H2SO4 will exist
predominantly as the protonated species. |
My calculation showed that no amount of HBr added can get H2SO4 predominantly protonated. I dont think there is any confusion.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ok... nix my HBr example if you don't like it. It is purely conceptual that if you have something with a lower pKa (strong acid or weak acid), it
will protonate other species which have a higher pKa. If HBr weren't volatile, this would be possible.
I do think that my calculation above demonstrates the point that in an infinitely dilute solution is when H2SO4 is most fully dissociated. Do you
agree?
[Edited on 24-2-2009 by Arrhenius]
|
|
|
len2
Harmless

Posts: 32
Registered: 13-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Arrhenius
Ok... nix my HBr example if you don't like it. It is purely conceptual that if you have something with a lower pKa (strong acid or weak acid), it
will protonate other species which have a higher pKa. If HBr weren't volatile, this would be possible.
|
My calculation shows that thats not true for strong acids in general.
| Quote: | Originally posted by Arrhenius
I do think that my calculation above demonstrates the point that in an infinitely dilute solution is when H2SO4 is most fully dissociated. Do you
agree?
|
Yes I agree
|
|
|
kmno4
International Hazard
    
Posts: 1502
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Hehe....
Practical problem:
We have: 0,01 mol/dm3 H2SO4 (let's say there is no HSO4(-) ion dissociation) and 0,01 mol/dm3 HBr.
Let's mix equal volumes of these solutions - what is pH of this mixture ?
ps. on the picture I attached you can see that H2SO4 is more acidic than HCl and even HClO4 (up to concentration ~7 mol/dm3).
Surely H2SO4 is also more acidic than HBr, not because of its volatility ....
In Brauer or IS there is described simple procedure for making HBr:
Dissolve 120g KBr in 200g H2O and cool. Next, slowly add 90 ml conc. H2SO4 keeping temp. below 75 C. Cool it to room temp. and filter off
KHSO4 crystals (solution is distilled to obtain HBr(aq) azeotrope)... 
[Edited on 25-2-2009 by kmno4]
|
|
|
len2
Harmless

Posts: 32
Registered: 13-9-2008
Member Is Offline
Mood: No Mood
|
|
pH ~ 2 if you neglect second deprotonation - which is a bad approx at this pH, but illustrates point.
The only import from H2SO4 having a pK = -3, and HBr -6 is that in dilute (c<0.1mol/L) solutions [H2SO4] is tiny (=c^2/1000<10^-5mol/L) while
[HBr] is 'tinier' by a factor of 1000. What happens at very low pH, i.e. in a medium where H2O is rare (or absent) is nothing to do with this, the
roles could very well be reversed.
[Edited on 26-2-2009 by len2]
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
A most interesting topic. The following is my own take on the matter – I cannot give authoritative literature references for some of it. So, IMHO,
(1) The concept of pH has nothing to do with water necessarily. In essence it means the negative logarithm to base 10 of the hydrogen ion
concentration [H+] (in a solvent), measured in mols per liter of solution volume. It is used as a convenience for the huge range that [H+] can assume
in a solution, from ~ 10^-15 to >1. There seems to be a misconception around that [H+] cannot exceed 1 mol/L for some reason and hence pH cannot be
less than 0. Of course it can – what would stop it? Hence conceptually under this definition, pH can range from ~15 down to some limiting negative
value –x<0.
(2) For a solvent to appreciably ionize a salt, base or acid, it must have a substantially large dielectric constant K. Such liquids are polarized,
according to simple electrical theory (Clausius/Messoti). Examples are ethanol (K~20), water (K~80) and sulphuric acid (pure, K~100). Non-polar
liquids generally have K~1.3-2 (e.g. benzene).
(3) For strong electrolytes disassociation into ions is considered complete (Arrhenius et al) but the effects of association with solvent and/or/
solute molecules can cause the effective ionization (activity) to be appear less than complete. (Debye,Huckel) – or even greater than one, due to
complexing. The activity coefficient γ is the ratio of apparent ionization divided by the concentration if fully ionized. The Debye-Huckel theory
can be used to about 0.1 M to give good results. Other corrections to the activity can extend this to around 0.5M ionic strength solution (e.g.
Davies).
(3) The activity coefficient can be estimated by means such as electrical conductivity measurements, freezing point depression, osmotic pressure etc.,
as taught in thermodynamics. The product of activity constant and total acid concentration, however, usually increases with concentration, and does
for sulphuric acid although the activity drops as low as 0.119 at 2 molal (2m=1.97M, 16.4% acid), but increases at higher concentrations (CRC). From
the same data the computed pH would go negative at ~ 4.2M.
(4) To complicate things further, as well as being diprotic, pure 100% sulphuric acid is autoprotolytic due to its very high dielectric constant. At
high concentrations in water, the predominate ion is HSO4-.
The autoprotolysis is said to be (Pauling, Gen. Chem):
2H2SO4 < - - >H2SO4. H+ + HSO4-
in which the hydrogen ion is attached to a H2SO4 molecule in a similar manner to hydronium ion, H3O+, to water. The ionization constant which we could
call ks (similar to water’s kw =~10^-14) is ~2*10^-4 = [H3SO4+][HSO4-]. The activity of H+ would then be ks^1/2 =1.414*10^-2, giving an pHs of 1.85.
So, somewhere between 10M and 18.6M (pure acid) H2SO4 does apparently increase in pH as defined as [H+] mol/liter. It there does have a least pH.
(5) Quoted measured pH is referred to an operational (practical) standard defined by such bodies as NBS (NIST), NPL, IUPAC etc. It is measured by the
open circuit voltage of a (two) reversible electrolytic cell(s). The physics of the device is such that a difference of 59.1 mv is produced for each
pH interval. However, this is only true at 25C. This voltage varies as absolute temperature T. Hence at 90C the slope of the device will be 363/298 as
much, about 22% higher. This represent an error in true hydrogen ion concentration of ~66%. You had better reduce your measured values to STP – yes,
pressure too. And the pH of water is 7.00, isn’t it? Sorry, the pH of water, under the definition in(1) above, varies from about 7.5 at 0C to 6.5 at
90C and so does the definition of ‘neutrality’. So the apparently simple concept of what pH means becomes very complicated on a more refined
study…
Regards to all critical readers, Der Alte
|
|
|
kmno4
International Hazard
    
Posts: 1502
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by DerAlte
(...) |
(1) and (5) - pH concept is reserved generally for water solutions at 25 C (only). Value of pH is between 0-14.
(4) It is in your dreams only. In reality, H<sub>0</sub> for H2SO4 at 20, 40, 60, 80, 100 % is:
-1,0; -2,4; -4,5; -7,3; -11,9
You can create and use your private system of chemical definitions, but who cares about them (but you) ?
[Edited on 2-3-2009 by kmno4]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by kmno4
Hehe....
Practical problem:
We have: 0,01 mol/dm3 H2SO4 (let's say there is no HSO4(-) ion dissociation) and 0,01 mol/dm3 HBr.
Let's mix equal volumes of these solutions - what is pH of this mixture ?
ps. on the picture I attached you can see that H2SO4 is more acidic than HCl and even HClO4 (up to concentration ~7 mol/dm3).
Surely H2SO4 is also more acidic than HBr, not because of its volatility ....
In Brauer or IS there is described simple procedure for making HBr:
Dissolve 120g KBr in 200g H2O and cool. Next, slowly add 90 ml conc. H2SO4 keeping temp. below 75 C. Cool it to room temp. and filter off
KHSO4 crystals (solution is distilled to obtain HBr(aq) azeotrope)... 
[Edited on 25-2-2009 by kmno4] |
Uhm, H2SO4 is simply not a stronger acid than HBr...
The reason you can make HBr from H2SO4 and KBr, is that KHSO4 seems to be insoluble in the very cold solution.
When it crystallises, the HSO4- is removed from the equillibrium reaction (well not completely, but it is not in solution and thus much less active),
and it shifts to the right, producing more HSO4-, wich crystallises as the potassium salt.
The fact that you can make acid A from acid B does not indicate acid B is stronger...
Other factors are also in the game, like votality. I have made a stream of HI fumes (HI is a very strong acid), by heating some KI crystals in 85%
H3PO4 (weak acid). Why does it work? HI is volatile, so this reaction shifts to the right:
H3PO4 + KI <<---->> HI + KH2PO4
Another good example is making HCl from NaCl and NaHSO4.
You can also move the equillibrium by precitipating one of the reactants.
As an example, just imagine perchloric acid would be a weaker acid than , say, HI. You would still be able to make HI by adding KI to perchloric acid
(<60%, otherwise oxidising), and filtering the very insoluble KClO4.
|
|
|
kmno4
International Hazard
    
Posts: 1502
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Indeed, H2SO4+KBr is not good example.
Generally, I ment that strong acids in water have the same strength (at relatively low concentrations) and no acid is protonated by another one. It
happens even if "K" constants differ many orders.
In the case of preparing HBr, there is 200g of water (more than 10 moles) and less than 3 moles of other substances - in this mixture water is not
rare. But a propos comparing acids' strength, it is a little "misleading" example. Of course, there is also reaction of Ba(ClO4)2 and diluted H2SO4
-> diluted solution HClO4; precipitating sulfides of metals from highly acidic solutions by H2S is another example. In these cases, driving force
of reaction can be forming hard soluble substances.
NaCl and NaHSO4 is rather bad example - reaction strats at 400 C or so and it generates no acid but HCl(g).
H3PO4 is not so weak at high concentrations. H<sub>0</sub> for 100 % H3PO4 (the only data I have for this acid) is -4,5 - the same
value as for 60 % H2SO4.
H<sub>0</sub> for 80% H2SO4 and 70 % HClO4 are almost the same (-7,3 and -7,7). Strong asids are not like weak acids and
10<sup>5</sup>-fold difference in "K" does not mean that HBr is stronger than H2SO4. "K" (in water) is very poor measure of acidity of
strong acids for any concentrations (in water).
|
|
|