chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
making a quaternary salt from secondary amine?
Well I'm trying to make a quaternary salt of a piperidine-like compound to use as a leaving group in a subsequent reaction.
I've looking up a lot of journal articles and have found the use of MeI in turning tertiary amines (I think it was tropane) into quaternary salts.
Could I just use excess MeI for making my secondary amine into a tertiary salt? Or I would I have to somehow deprotonate it before doing so? I'm not
exacty sure how that hydrogen will effect the methylation.
Also, would iodoethane give similar results? (in making a quat. salt that is a good leaving group?)
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Both would work, you need to add a base as well (preferably non-nucleophilic).
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
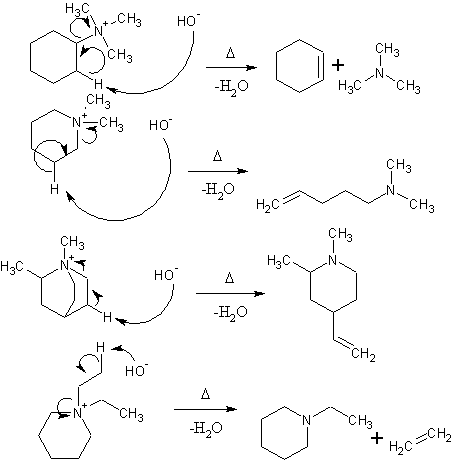
This largely depends on what you are trying to do with this reaction. If you are trying to get N-methyl piperine or N-methyltropane to leave during a
hoffman elimination, you will probably end up cleaving the piperine/tropane system instead. I don't think iodoethane is a good choice since it may
eliminate preferentially (depending on what else is attached to the nitrogen) and give ethylene. I believe that iodomethane is used intentionally
because the methyl cannot eliminate to give an unsaturated compound.
More specifics would be extremely helpful.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
It takes two moles of methyl iodide to form the quaternary salt in the case of a heterocyclic amine  Water is the only product of the Hoffman elimination in this case, unless it's repeated to obtain the olefin and
trimethylamine Water is the only product of the Hoffman elimination in this case, unless it's repeated to obtain the olefin and
trimethylamine 
Chemistry is our Covalent Bond
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Hoffman elimination procedes with the formation of the least substituted alkene. If you were to use bromoethane, as shown in the last reaction,
elimination of one of the ethyl groups to ethylene is an unhindered path that the reaction may take. I don't know if the abstraction of a proton from
a methyl carbon is any more favorable than the cleavage of the 6-membered ring (and abstraction of the proton from a methylene group). If it is more
favorable, the reaction will proceed mainly via elimination of ethylene. If not, you will primarily cleave the ring to give
N,N-diethyl-pent-4-en-1-amine. If methyl groups are used, there is no beta-carbon to abstract the hydrogen from except for the ones on the ring and it
must proceed with ring cleavage.
[Edited on 11-9-08 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm troubled. You plan to use a piperidine-like compound and the N center is part of a leaving group? This sounds like a really wasteful thing to
do. Please enlighten us further or at least u2u me with some details.
[Edited on 9-11-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
I'm not trying to do a hoffman elimination.
chemrox: this is what confused me about the reaction too at first but there is literature out there using tropinone compounds, thats where my main
concern came from since the tropinones already have a methylated N, so adding MeI just adds another making the quat. I'm curious if my secondary, with
the hydrogen, will work the same or if I need to abstract the hydrogen first with like LAH(or milder)?
The rxn I ultimately want to do is to take this quat. salt and react it with a primary amine, my understanding is that the charged nitrogen is
somehow eliminated and the primary amine takes its place.
I have 3-chloropiperdine, I want to methylate it to the quat. salt and react it with 3-phenylpropylamine. The product should be the addition of the
phenylpropylamine adding to where the quat salt was.
I can pull up the original tropinone paper if you are still having questions
Edit: I'm really just interested in how to make the quat salt since I know the other chemistry works, but I'm worried about that hydrogen.
[Edited on 9-11-2008 by chemchemical]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Please post that paper!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemchemical
I'm curious if my secondary, with the hydrogen, will work the same or if I need to abstract the hydrogen first with like LAH(or milder)?
|
The acronym "LAH" is generally used for LiAlH4. May I ask where it came into being mentioned? What does it have to do with such a simple N-alkylation?
You can make a quat out of a secondary amine by simply refluxing the amine, 2eq alkyl halide and NaHCO3 in ethanol, filtering off the insolubles and
rotavaping (with further purification if necessary). There are numerous other examples in the literature.
However, your more than obvious lack of basic understanding of concepts like acid/base chemistry, nucleophilic substitution and other such, makes it
highly doubtful that you actually know what you want to do and what you are talking about.
| Quote: | | The rxn I ultimately want to do is to take this quat. salt and react it with a primary amine, my understanding is that the charged nitrogen is
somehow eliminated and the primary amine takes its place. |
It appears to me that you completely misunderstood some particular reaction. What you describe sounds like a Hoffman elimination/Michael addition
tandem reaction, but since you did not provide a single reference, this thread is quite unreadable (and that is the reason why I'm moving it to
Beginnings section).
| Quote: | | I have 3-chloropiperdine, I want to methylate it to the quat. salt and react it with 3-phenylpropylamine. The product should be the addition of the
phenylpropylamine adding to where the quat salt was. |
You can not make N,N-dimethyl-3-chloropiperidinium salts for the more than obvious reasons.
| Quote: | | Edit: I'm really just interested in how to make the quat salt since I know the other chemistry works, but I'm worried about that hydrogen.
|
Huh!?
You don't understand basic proton transfer chemistry, but you know all the rest about organic chemistry? 
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
side notes
Here are a couple of articles you might get something out of. I do rather agree with Nicodem that you have some of the concepts and terms mixed up
and also suggest reading a book on mechanisms and reactions. I think his reply was a bit harsher than it had to have been but his points were
correct.
Attachment: eq-Hutchinson & Tarbell.pdf (600kB)
This file has been downloaded 754 times
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
and here's the other one
Attachment: Analgesics- Patchett & Giarrusso.pdf (360kB)
This file has been downloaded 787 times
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nicodem
Huh!?
|
Alright, well a little searching and I was able to find out that I can methylate the piperdine twice (one pot) to make it a quaternary salt. They go
from 22 (piperdine derivative) to 23 (a piperidinium iodide with excess MeI. My molecules are simpler too.
Attachment: dimethylation piperine.pdf (874kB)
This file has been downloaded 779 times
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
Please post that paper! |
Here is the paper on the tropinone derivatives, the reason I wanted to know if it was possible to make the quaternary form. Even though piperidine is
lacking that carbon bridge, it should still work, probably even better since there is less hindrance.
Attachment: Tropinone.pdf (207kB)
This file has been downloaded 1471 times
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemchemical
Well I'm trying to make a quaternary salt of a piperidine-like compound to use as a leaving group in a subsequent reaction.
I've looking up a lot of journal articles and have found the use of MeI in turning tertiary amines (I think it was tropane) into quaternary salts.
Could I just use excess MeI for making my secondary amine into a tertiary salt? Or I would I have to somehow deprotonate it before doing so? I'm not
exacty sure how that hydrogen will effect the methylation.
Also, would iodoethane give similar results? (in making a quat. salt that is a good leaving group?) |
Well, I guess thats solved. MeI will make the iodide salt, I still do not know if iodoethane would work, I'm pretty sure I saw a tropinone with one
ethyl and one methyl.
As the author states: "The supposed mechanism consists in the elimination
of dimethylamine yielding cycloheptadien-2,6-one
in situ, followed by addition of primary amine to the
diene to afford the desired tropinone derivative."
| Quote: | Originally posted by Nicodem
The acronym "LAH" is generally used for LiAlH4. May I ask where it came into being mentioned? What does it have to do with such a simple N-alkylation?
You can make a quat out of a secondary amine by simply refluxing the amine, 2eq alkyl halide and NaHCO3 in ethanol, filtering off the insolubles and
rotavaping (with further purification if necessary). There are numerous other examples in the literature.
However, your more than obvious lack of basic understanding of concepts like acid/base chemistry, nucleophilic substitution and other such, makes it
highly doubtful that you actually know what you want to do and what you are talking about. |
It appears that you misunderstood me. When I said LAH I meant lithium aluminum hydride. I incorrectly thought you might need something to abstract the
proton, something like a base, such as the NaHCO3, or baking soda, for it to work. I was getting the methylation of nitrogen confused with oxygen for
some reason as the methylations I have done all have been with oxygen.
I really dont understand why chemists appear to be so arrogant, I mean is my lack of basic understanding really that obvious?
I was talking about abstracting a proton with LAH.
I know nitrogen is acting as a nucleophile to kick out the dimethylamine, I wasnt sure exactly how it did that or could do that with a cyclic though.
As for the other such I dont know... I'll work on it.
I said what I wanted to do, make the quat salt and react it with a primary amine like they do in the tropinone paper I posted. My question has been
answered by the other paper I posted. Sorry if you didnt understand me.
And yes, I do not know proton chemistry but I am a specialist in anything organic 
I guess I have a lot to learn in grad school 
[Edited on 14-11-2008 by chemchemical]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
LiAlH4 is a strong reducer, so rarely used to deprotonate a compound. Strong bases like NaH or t-BuOK are rather used. Most N-H protons require such a
strong base except if they are particularily acid (sulfonamides for example) to be completly deprotonated, but with alkylating the very nucleophilic
amines with a free doubet you do not need to deprotonate like you would with a alcohol, the base is just there to capture the HX formed, so things
like K2CO3 can be used. Without any base, the amine would be alkylated anyhow, but the more basic unreacted amine would form the HX salt, which cannot
be alkylated as it doesn't have a free doublet anymore. If a mild base is added, the freebase is liberated can can be alkylated again.
So most amines only require a alkylating agent and a mild base in a suitable solvent. Hindered amines can require long reflux, especially with larger
electrophile.
The doublet in amides in much less "avaible" though, becasue of the mesomerism with the C=O, so it cannot attack an electrophile as readily. The N-H
in a primary or secondary amide will have to be deprotonated for the alkylation to happen. The formed anion is also stabilized by mesomery, and can
react very easily with an electrophile. Strong bases will be used to entirely deprotonate the amide, or in certain case a milder base like KOH can be
used to partially deprotonate the amide.
Phenols can be alkylated with light base like K2CO3, as they are particularily acid for R-OH compounds. So only a small amount is deprotonated
(equilibrium), but get immediatly alkylated. The phenolate is consumed, so more phenol gets deprotonated, shifting the equilibrium until all has
reacted. Usually excess base is used.
On the other hand, if KOH or another strong base is used, all the phenol is deprotonated, and the very nucleophilic phenolate anion is quickly
alkylated.
Hope this helps.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemchemical
Well, I guess thats solved. MeI will make the iodide salt, I still do not know if iodoethane would work, I'm pretty sure I saw a tropinone with one
ethyl and one methyl.
As the author states: "The supposed mechanism consists in the elimination
of dimethylamine yielding cycloheptadien-2,6-one
in situ, followed by addition of primary amine to the
diene to afford the desired tropinone derivative." |
N,N-Dimethyl-3-chloropiperidinium salts, regardless of the impossibility of making them from 3-chloropiperidine, can not go trough such an
elimination/Michael addition tandem reaction for the simple reason because they don't have a beta-carbonyl group. Therefore, there can be no easy E1cB
double elimination step and since no enone forms there can be no Michael addition of any kind either.
| Quote: | | It appears that you misunderstood me. When I said LAH I meant lithium aluminum hydride. I incorrectly thought you might need something to abstract the
proton, something like a base, such as the NaHCO3, or baking soda, for it to work. |
LiAlH4 is lithium aluminum hydride!
R<sub>3</sub>N + RX => R<sub>4</sub>N<sup>+</sup> + X<sup>-</sup>
As you see, no proton transfer is involved in the quarternisation of tertiary amines with alkyl halides - so no base is needed.
The base is only required to deprotonate the intermediate tertiary amine salt when starting from secondary amines (like in what you want). Their pKa
is about 9 so just about any base of comparable strength and low nucleophilicity is enough.
R<sub>2</sub>NH + RX => R<sub>3</sub>NH<sup>+</sup> + X<sup>-</sup> ={base]=>
R<sub>3</sub>N
Besides, if hypothetically it was possible to quarternize 3-chloropiperidine this way (which is not), you would need to use an additional equivalent
of the base since 3-chloropiperidine can only be stored as its salt (like its hydrochloride; but it is not even commercially available so what do I
know what form do you have since you arrogantly don't say).
| Quote: | | I really dont understand why chemists appear to be so arrogant, I mean is my lack of basic understanding really that obvious? |
It was you who started arrogantly by not even bothering telling what you want and even so much arrogant as not to provide the reference. Not to
mention your not only arrogant but also dumb claim you know not about the proton transfers which are the base of basis of most polar mechanism based
organic chemistry, but knowing all the rest of organic chemistry involved (which you now showed you don't have the slightest clue either).
Now you act surprised if you get an equally arrogant reply? Just read again what you write here:
| Quote: | And yes, I do not know proton chemistry but I am a specialist in anything organic  |
WTF?
| Quote: | I guess I have a lot to learn in grad school  |
You have to learn to learn first.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nicodem
N,N-Dimethyl-3-chloropiperidinium salts, regardless of the impossibility of making them from 3-chloropiperidine, can not go trough such an
elimination/Michael addition tandem reaction for the simple reason because they don't have a beta-carbonyl group. Therefore, there can be no easy E1cB
double elimination step and since no enone forms there can be no Michael addition of any kind either. |
Does the impossibility lie in the halide? I should not have used that as an example I suppose, I have other piperidine analogues with non-halide
substituents. The paper I posted has a piperidine analogue being turned into the N,N-dimethyl-piperidinium salt with MeI. So it is possible to turn a
piperidine into the dimethyl salt. Its in the paper.
I said before that I was never trying to do a Micheal addition. I'm going to have to assume that the reaction is not going through a Micheal addition
then, since none of the components of a Michael addition are present (double bonds, carbonyls).
Yes, I did want an addition but someone else thought it was Michael.
Please elaborate on your claim that chloropiperidine will not work. I was using it as an example analogue.
| Quote: | Originally posted by Nicodem
It was you who started arrogantly by not even bothering telling what you want and even so much arrogant as not to provide the reference. Not to
mention your not only arrogant but also dumb claim you know not about the proton transfers which are the base of basis of most polar mechanism based
organic chemistry, but knowing all the rest of organic chemistry involved (which you now showed you don't have the slightest clue either).
|
It appears we have had a miscommunication, I forget english isnt everyones first language. I have provided the references now so thats not an issue.
Your replies do tend to have a bite to them though, I felt it demeaning which explains the adjective I used.
My very first post I said what I wanted, other people added their own opinions. All I wanted to know was if I could methylate piperidine twice without
a base... sorry if the following quote sounded arrogant. I didn't realize people would have so much trouble with this question:
| Quote: | Originally posted by chemchemical
Could I just use excess MeI for making my secondary amine into a tertiary salt(I meant quaternary)? Or I would I have to somehow deprotonate it before
doing so? I'm not exacty sure how that hydrogen will effect the methylation. |
| Quote: | Originally posted by Nicodem
Now you act surprised if you get an equally arrogant reply? Just read again what you write here:
| Quote: | And yes, I do not know proton chemistry but I am a specialist in anything organic  |
|
Sorry, but that was my attempt at using sarcasm against your original claim that I knew everything organic but nothing protonic. I was trying to get
some basic answers when you posted that "harsh" (another posters words) reply.
I really do not know that much ochem besides undergrad level. I will be taking graduate level ochem though next year at a top ten research Uni in the
USA, so I should learn a lot that year.
....
So I provided the references, my question is answered and we now know it is possible to dimethylate piperidine and then react it with a primary amine,
which goes through some unknown addition/elimination reaction
I didn't mean to offend anyone, but I didn't think I deserved such a harsh reply. There were obviously some miscommunications in this post, probably
from my poor use of the english language and others to comprehend it.
[Edited on 19-11-2008 by chemchemical]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemchemical
| Quote: | Originally posted by Nicodem
N,N-Dimethyl-3-chloropiperidinium salts, regardless of the impossibility of making them from 3-chloropiperidine, can not go trough such an
elimination/Michael addition tandem reaction for the simple reason because they don't have a beta-carbonyl group. Therefore, there can be no easy E1cB
double elimination step and since no enone forms there can be no Michael addition of any kind either. |
Does the impossibility lie in the halide? I should not have used that as an example I suppose, I have other piperidine analogues with non-halide
substituents. The paper I posted has a piperidine analogue being turned into the N,N-dimethyl-piperidinium salt with MeI. So it is possible to turn a
piperidine into the dimethyl salt. Its in the paper. |
3-Chloropiperidine is a beta-haloamine and as such is unstable as a free base where it is in equilibrium with the corresponding aziridine
hydrochloride which is also unstable and will tend to form polymerization products. In any case you can not selectively N-methylate it before
an intramolecular reaction occurs.
Obviously you can turn other piperidines without such sensitive groups to their quaternary salts, that is no problem and there are hundreds of papers
with hundreds of examples in the literature. What you fail to understand is the mechanism of the reaction of the next step. You don't get it that for
an E1cB elimination you need a carbonyl group attached at the beta position from the amine. Without this carbonyl there can be no Michael addition and
therefore your desired reactions does not work.
Why don't you read your referenced paper again? Also, read some others on the same tandem reaction - there are several since it is a relatively well
known reaction. Perhaps all this would make more sense to you if you would not limit yourself in your learning process.
| Quote: | I said before that I was never trying to do a Micheal addition. I'm going to have to assume that the reaction is not going through a Micheal addition
then, since none of the components of a Michael addition are present (double bonds, carbonyls).
Yes, I did want an addition but someone else thought it was Michael. |
You are a bit ambivalent here. You say you are not attempting a Michael addition yet the paper you posted is about the double Michael addition on the
dienone intermediate.
| Quote: | | All I wanted to know was if I could methylate piperidine twice without a base... |
Of course you can, after all piperidine is a base itself, however the yields will suffer. It is unlikely you can get more than 30-50% yield this way.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Oboe
Harmless

Posts: 6
Registered: 30-10-2008
Member Is Offline
Mood: No Mood
|
|
Related theoretical question
Hi, I have a related theoretical question with the methylation of a primary amine in methanol with MeI.
Equation is
R-NH2 + 3MeI goes to R-NMe3I + 2HBr
My question is whether to use 2 moles of base or excess? and also if to use excess MeI also for good yield?
Not very good with practical chemistry  I would guess to use excess of both for
good yield of quaternary compound? I would guess to use excess of both for
good yield of quaternary compound?
Also what base to use? potassium carbonate dissolves well in methanol?
[Edited on 26-11-2008 by Oboe]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, definitively use excess base (5eq are not unusual), and a slight exces of MeI ( ~1.25 eq). The reaction should proceed pretty well unless your
primary amine is in someway hindered, but a few hours reflux should be perfect.
K2CO3 is an excellent choice, and it does not need to be soluble in the solvent. It will form a suspension (be sure to dry it and to powder it before)
and will gradually be consumed, although there usually isn't that much of change in appearance.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
chemchemical
Harmless

Posts: 31
Registered: 30-8-2007
Member Is Offline
Mood: No Mood
|
|
Posted below is the link to the paper on the double N-methylation of a piperine derivative, I posted it before in this same thread.
I would like to perform a similar reaction using 4-piperidone, the monohydrate chloride. My goal is to isolate the N,N-dimethyl iodide, not to perform
any eliminations or additions.
My question is, do I need to isolate the freebase before I perform this reaction? No base is being used in the cited reaction.
http://www.sciencemadness.org/talk/files.php?pid=141118&...
"Amine 22 (550 mg, 2.4 mmol) and a large excess of
Me1 (1.5 mL) in 20 mL of MeOH/CHzCl2 2/1 were stirred at room
temperature for 24 h, then Et20 (50 mL) was added. A solid was
filtered and recrystallized from MeOH (450 mg, 48.8 %),"
[Edited on 6-4-2009 by chemchemical]
|
|
|