olivertwist
Harmless

Posts: 2
Registered: 15-6-2007
Location: us
Member Is Offline
Mood: No Mood
|
|
i need some help with resonance
hi all. i'm having a little trouble with an o chem problem for school. we have to draw all the resonance structures for p-dimethylaminophenol and
p-nitrophenol. i think i'm on the right track, but do i start moving electrons from the OH group toward the N or the other way? any help would be
great.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
The way I looked at it wasn't as 'electrons moving' but rather, electron density.
There are a few special cases where electrons or electron pairs really do move around a molecule, but in most cases of resonance, I
<i>think</i> that they don't move.
Resonance drawings are not 'real', but more a thought exercise. It shows you the overall electron density as a combination of the acceptable resonance
structures. Think of it as a method for overcoming the weakness of simple lewis structure drawings in organic (oh, and inorganic) chemistry.
I remember being reminded that 'electron pushing' a'la reaction mechanisms are not strictly true, but still are useful in predicting a mechanism.
So in your case, both the alcohol and amine/nitro groups are electron donating. You'll get a combination of benzenes with a delocalised negative
charge from the amine/nitro and alcohol.
p.s. Yes, I was both rewarded and reviled for my creative use of dotted lines, curved 'multi-bonds' and some shading in org.chem.
Caveat Orator
|
|
|
olivertwist
Harmless

Posts: 2
Registered: 15-6-2007
Location: us
Member Is Offline
Mood: No Mood
|
|
thanks for your help. i know that the structures aren't "real" but i still have to be able to draw them for the test. i also understand that they will
delocalise but for the purpose of the test, do you start at the -OH end or the N?
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
All you have to remember is that for the purposes of "electron pushing" you have to 1) identify which groups are electron rich and electron poor; and
2) push electrons in the direction of "electron rich to electron poor."
So, for the example of p-nitrophenol, you start with those lone pairs in the hydroxy group...
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
This should give some idea about how resonance works
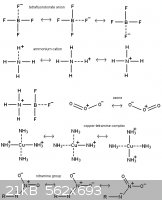
[Edited on 12-11-2011 by AndersHoveland]
|
|
|