PerKhlorHate
Harmless

Posts: 13
Registered: 23-11-2007
Location: Right behind you holding a knife poised to slash y
Member Is Offline
Mood: radioactive
|
|
Fluorates?
logic dictates that because there is XClO3 there should be XFO3 oh what big oxidizers you have!
Edited title. chemoleo
[Edited on 2-1-2008 by chemoleo]
\"I Reject Your Reality and Substitute My Own\"
-Adam Savage
|
|
|
chloric1
International Hazard
    
Posts: 1146
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Hmmm! Don't never hear fluorate mentioned either in old or new chemistry texts. Some texts even state that attempts to make or isolate fluorate have
been unsuccessfull. So, I guess this means you don't read.
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
I guess you mean fluorate!
A quick search......
"The fluorate ion, FO3–, has also never been detected, even though analogs containing the other halogen elements are well known. The problem here
may well lie with the very small fluorine atom, which would allow the oxygens to approach so closely that they would repel each other."
I guess it's the same reason you don't get pernitrates. But perborates and percarbonates exist and boron and carbon are smaller than nitrogen,
strange! (Not in a covalent bond, though, see below!)
Edit: Covalent radii, Cl = .99, F = .71, N = .73, C = .77, B = .79 and O = .74 I'm guessing, but this would suggest an element needs a covalent radius
greater than oxygen to allow (per)X-ates to form!
[Edited on 1-1-2008 by Xenoid]
[Edited on 1-1-2008 by Xenoid]
|
|
|
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
For what (little) it's worth, here is my guess.
Note that, in a halogenate, the halogen is in the +5 oxidation state and
has valence 5 (two double-bonded oxygens and a single bond to
another oxygen). However, fluorine is at the top of the periodic table
and has only 9 electrons, two of which are located in the inner n=1 shell.,
leaving 7 in the outer shell, so +5 oxidation sounds like a tall order.
As for perborates and percarbonates, the boron and the carbon in them
have valence 4. So I guess the reason you don't have perfluorates is
that the fluorine has too few electrons be forming all those bonds in
a stable molecule.
Now that you mentioned nitrogen, however, this explanation sounds a bit
less convincing because nitrogen is in the same row with fluorine but
it can form high oxidation states and have valence 5 (e.g. NH4Cl) so it
isn't that simple.
|
|
|
PerKhlorHate
Harmless

Posts: 13
Registered: 23-11-2007
Location: Right behind you holding a knife poised to slash y
Member Is Offline
Mood: radioactive
|
|
urr umm maybe hypofluorite? FO-
\"I Reject Your Reality and Substitute My Own\"
-Adam Savage
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
The only realistic structure for the hypothetical fluorate would be this:
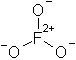
as it is the only way you're going to get three oxygens bonded to one fluorine atom (you can't get a structure completely analagous to chlorate,
because fluorine lacks d orbitals of low enough energy to participate in bonding, and thus cannot form hypervalent compounds). And what an hideous
structure it is. First off, you have a formal charge of +2 on the fluorine, which being the most electronegative element there is, isn't going to like
that terribly much. Couple that with all of the oxygens having a formal negative charge, and you've got some very unhealthy charge separation going
on.
Another way of thinking about it is that fluorine is a weaker Lewis base than oxygen. While oxygen will happily donate one electron pair to an
electrophile (for instance, to produce H3O+), it is pretty much unheard of for it to donate two (i.e. no H4O++). If oxygen is unwilling to donate two
electron pairs, what are the chances of fluorine donating two electron pairs?
If you were to (somehow) get a fluorate ion, it would almost certainly immediately decompose, liberating oxygen:
O3F- -> OF- +O2
[Edited on 2-1-2008 by Pyrovus - replaced that ghastly ASCII diagram]
[Edited on 3-1-2008 by Pyrovus]
Never accept that which can be changed.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Hypofluorites exist though they are tricky to isolate, or at least the hypofluorous acid is, I remember reading about it in a paper that covered the
preparation of compounds that were once thought impossible.
In fluorates, the fluorine would have to carry either a formal charge of +5 (not gonna happen with fluorine being as wickedly electronegative as it
is) or -6 forcing the oxygen molecules to be +2 each. Just look at the stability of the fluorine oxides, oxygen doesn't like to be put in that
position.
Anyway, it's not really logic there, just a pattern and it just doesn't hold for fluorine.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The funny thing about the halogens is, fluorine isn't one of them. 
Tim
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
How are you ever going to get a positive charge on the fluorine?
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by PerKhlorHate
urr umm maybe hypofluorite? FO- |
Duh...! Have you not heard of Google? Try it, you'll get over 5000 hits.. 
Including such gems as pentafluorotellurium hypofluorite.
and statements like;
"Hypofluorite compounds are well known and find utility in a wide variety of industrial applications. They are especially useful as fluorinating
agents for introducing fluorine atoms into another compound, and as intermediates in various synthetic reactions."
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
According to Wikipedia (I know not the best source) hypofluorous acid is the only hypohalic acid that can be isolated as a solid.
I always thought it would be nearly impossible to form hypofluorous acid and have it last for more than a few seconds.
"Treating phenanthroline with this reagent yielded the previously elusive 1,10-phenanthroline dioxide"
Very interesting stuff.
And since when is F not a halogen? Did I miss something? I always found Astatine, the most elusive of the halogens, interesting.
[Edited on 2-1-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
chloric1
International Hazard
    
Posts: 1146
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I think Tim was talking more about how fluorine differs so greatly from all the other halogens. Brauer's text is even layed out treating fluoride
compounds separaterly from others.
Fellow molecular manipulator
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
IIRC hypofluorous acid is made by leading fluorine through freshly wetted teflon helices and condensing the vapors in dry ice and liquid nitrogen
traps where the HOF freezes. Early methods that detected it in trace quantities passed elemental fluorine over a wet porous surface or something of
that nature.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The reasons why F is so reluctant to form any compounds in which it has an oxidation state of -1 are:
(a) because it is the most electronegative of all elements that can form actual stable and isolatable chemical compounds (Ne and He may be
theoretically more electronegative if they could be induced to form Ne+ and He+ cations,, but they cannot form stable and isolatable chemical
compounds, although short-lived ions like HeH+ and NeH+, formed by passing sparks through mixtures of the gases, have been identified by mass
spectrometry); and
(b) because to form any compounds in which it has a valence other than -1 (formed by gaining an electron to form a completed electron octet, 2s2+2p6,
with sp3 hybridization of electron pairs, like Ne), it has to EITHER lose an electron firstly to become F+ in the +1 oxidation state (which is very
difficult, occurring virtually only on the decomposition of the KrF+ cation in [KrF][SbF6] (the F+ can then be made to react with NF3, ClF5, or BrF5
to oxidise these to NF4+, ClF6+, BrF6+, of which salts have been isolated, and OF3+ should also be possible although it has not been isolated), OR,
bonding covalently, to somehow expand the number of valence-band electrons beyond 8 by utilizing 3s and 3p electrons, which cannot be done because the
energy levels of electrons in the 3s and 3p orbitals are too much higher than those of 2p orbitals.
[Edited on 4-1-08 by JohnWW]
|
|
|