| Pages:
1
2 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Xenoid,
I edited the post above and stuck two links on it.
If you read the Part (I) paper, end of page 29, above it states:
_____________________________________________
In effect, platinum forms an equilibrium or quasi
equilibrium state when evolving chlorine, and
a rather different state for oxygen evolution.
During cojoint oxygen and chlorine evolution,
which occurs in dilute brine, there is interference
in the formation of surface layers and this
leads to accentuated metal dissolution.
_____________________________________________
This is an explanation of why erosion is bad in a Chlorate cell which is 'turning' into a Perchlorate cell (or in a Perchlorate cell with Chloride).
Both Oxygen and Chlorine are being made. Bad, bad, bad for Pt.
You can always not coat the first (say) four inches of the Anode so that no Pt is above solution level if erosion is bad in the headspace.
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Well, Dann2, I had another attempt at Pt on Ti, this time a piece of Ti mesh. The mesh (9 cm x 2 cm) was brushed heavily with a steel wire brush
(important) and placed in a tube of hot, concentrated HCl, When the HCl had turned violet (due to TiCl3) the mesh was removed and placed on a paper
towel. Within seconds, I had it fully painted with the Pt lustre resin. This was baked as described above and another 4 coats of Pt added, it looks
pretty good!
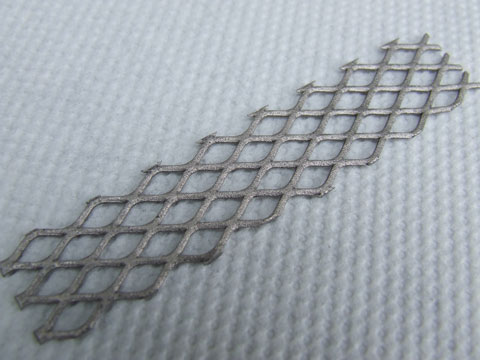
The anode was placed in a 150 ml saturated NaClO3 cell and operated at about 4V / 2.5A, within 10 mins. I could see the part of the anode in the
headspace area turning blackish. The anode part in the solution seemed stable so I left it running, it was OK about an hour later, but when I came to
check it 3 hours later the Pt had completely eroded away and the electrode had passivated. Perhaps it requires a more sophisticated etching procedure,
but I'm not sure I can do anything better. It has certainly not performed as well as the Pt coated MMO.
I have had a look on-line for suppliers of bright platinum lustre, and it now seems to be difficult to find, what is available is bloody expensive, it
would be cheaper just buying the ready-made electrodes from De Nora!
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Darn, Xenoid, I am sorry it did not work. That anode DOES look really good!
It may be the oxygen in your oven. Could you possibly seal it better and purge with argon before baking? Or perhaps a bit of charcoal or some other
agent could scavenge the O2 in the container.
One other option would be a stainless foil envelope. Stainless foil is commonly used to heat treat air-hardening steels, and does an outstanding job
of inhibiting oxidation of the contents. I don't know how fragile the unbaked coating is, but it might work.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I hate to resserect an old thread, but I'm getting ready to attempt this myself, only I'm going to use the method in dann2's patent. Question, is
67.2% Nitric acid and 31.2% HCl sufficient concentration to dissolve my scrap platinum into solution? I can get some higher grade HCl, but is it
necessary? I'll be using a heat treat furnace with an argon tap so ambient O2 shouldn't be a problem. One of the problems xenoid may have been
having is that platinum glaze is supposed to dry on it's own before firing. The method in the patent doesn't use the glaze, but the chloroplatinic
acid instead along with the reducing agent. I'm waiting for my furnace to get here now and have about 14 gms. of platinum scrap in the form of a
garbage disposaled ring and 5 grms. of 99.5% platinum wire. Blasted lab/shop can't get done soon enough.
[Edited on 3-6-2012 by hyfalcon]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Yeah! Way to go hyfalcon!
I'm afraid I never followed up on this process because I lacked a proper temperature controlled furnace. I also acquired a commercially made Pt -
coated anode and a largish solid Pt anode (described elsewhere - "Thoughts on Anodes" thread I think) for making perchlorate. I am sure this thermal
deposition of Pt on Ti is "do-able" for the amateur with temperature control and the right etching technique.
Please keep us informed, I look forward to seeing your results.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I picked up 4 more pieces of lasarred's mmo just so I had some to play with. I'm gonna cut and slice some of this to experiment with. I'm going to
try a coating over the mmo on one piece and I'll strip the mmo on the other one before etching and coating as per the patent. We'll see. I just wish
this oven was able to do a nitride run, but that's a whole different kettle of fish from argon.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Any results from this, hyfalcon? laserred's MMO seems to be used in that the stuff I've gotten has had a coating of some unknown residue. This is
easily stripped in dilute HCl, turning the acid bright yellow, but the MMO coating itself remains undisturbed. To verify this, I left a sample
overnight in concentrated HCl, and the MMO survived fine.
My point is that I'm thinking the laserred MMO material should probably be chemically etched/cleaned prior to any plating attempt. I did so for my
lead dioxide attempts, and that portion of the anode that was coated with MMO retained the LD coating very well when compared to the bare Ti areas,
particularly the saw-cut edges, which shed lead dioxide in large chunks.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Just got notification that my oven is ready for shipment. I started spreading gravel today for my new detached garage/shop. I've been fighting with
the county to get permits. I hate local government.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@ Xenoid (assuming your still reading my ramblings!) Have you seen any sign of surface wear on the Pt Anode (the one made from the crucible)?
Do you try to keep Chloride out of your perchlorate cells as much as possible. How many hours would it have clocked up and at what current densitys?
@hyfalcon
To hell with the silly gravel/garage/shop and rowing with the local politicians.
GET ON WITH THE ANODES 
Dann2
[Edited on 15-6-2012 by dann2]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Well I would except that's where I intend to do all the experimenting. After all the detritus gets stored in the attic of my new 30 X 40 work space,
I intend to set up my lab in the downstairs of the new building. Only disadvantage I'm going to have is I'll have to set up some sort of water tank
and drain tank for any water supply. The building is going to be dry. If I think I'm having problems with just a building and electrical permit, you
add plumbing into the mix and it gets REAL interesting.
|
|
|
| Pages:
1
2 |