Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Product prediction
I am having trouble predicting the product for the following reactants
HOOC-(CH2)2-COOH (aq)+ NaOH(aq)>>>>> ?
Is it a trick question the lecturer has given us.?
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
HOOC- and -COOH are carboxylic acid groups. What happens when an acid combines with a base? And what does that look like in this case?
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
I can see the H2O and possibly O2 and H2 and a Na salt with be produced it's the Methyl group that has me confused.
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
I'm guessing we get just methylene CH2(g) + H2O(aq)+ CO2(g)+ NaOH(aq)
[Edited on 16-1-2015 by Muzz1969]
[Edited on 16-1-2015 by Muzz1969]
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
What you have is a simple saturated dicarboxylic acid COOH-CH2-CH2-COOH. 1 mole of this will react with 2 moles of NaOH to give the sodium salt and
water thus :-
COOH-CH2-CH2-COOH + 2NaOH . COONa-CH2-CH2-COONa + 2H2O
The acid looks like butanedioic acid or succinic acid.
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Right so once the ph hits the neutral range you end up with a salt and water which when evaporated would leave your sodium salt .
Can anyone else confirm that this is the correct answer
[Edited on 16-1-2015 by Muzz1969]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Yes, it is correct. Remember that carboxylic acids are relatively easy to deprotonate because the resulting anion is resonance stabilized. This makes
that hydrogen more acidic and easier to remove.
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Right I get it now, so because the methyl group has a full orbital shells it stay non reactive is that correct ?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Muzz1969  | Right I get it now, so because the methyl group has a full orbital shells it stay non reactive is that correct ?
|
There's no methyl group (R-CH3) present in HOOC-(CH2)2-COOH. The part in red is an ethylene bridge
(-CH2-CH2-) between the two carboxylic acid groups. As nezza pointed out, this is a dicarboxylic acid and is known as butanedioic acid. (sometimes
called "succinic acid")
The reaction itself is just a simple acid-base neutralization. Assuming two moles of NaOH is used, it produces two moles of water and one mole of
sodium succinate (NaOOC-(CH2)2-COONa). The reason the two hydrogen atoms on the ends get removed is because the negative charge that results from
deprotonation becomes delocalized across the carboxyl group. The two oxygens "share" the negative charge, stabilizing the anion through a concept
known as resonance. Since the charge is distributed between the two oxygen atoms, neither really carries a true -1 charge, making the anion a pretty
weak base. Because of this, it's not only easier to pull the positively-charged proton away from those oxygen atoms, they're also less likely to grab
another proton later on and become an acid again.
The other four hydrogens, the ones on the ethyl chain, aren't nearly as acidic or easy to remove. The electron density in those carbon-hydrogen bonds
is, for the most part, being shared evenly, making them much more difficult to break. With that said, the carboxyl groups will slightly weaken those
adjacent C-H bonds by pulling some electron density away from hydrogen, so they actually are possible to remove if you use a strong enough
base. With NaOH, however, they just aren't acidic enough. (not to mention the aqueous conditions) In this particular case, though, removing one would
be difficult anyway, as you'd literally have to deprotonate the molecule three consecutive times before you'd actually get an alpha-hydrogen off, and
each deprotonation would be more difficult than the last.
Anyway, if you're more of a visual person, here's what's basically going on in your reaction. I didn't bother showing the deprotonation of the second
carboxylic acid group, but it's the same as the first.
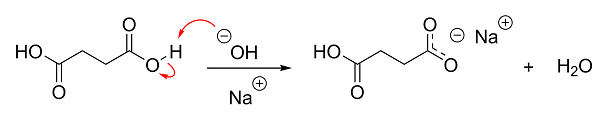
[Edited on 16-1-2015 by Darkstar]
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Yes , thanks darkstar the visual made it much easier to understand. I'm doing a bridge ing course at the moment to prep for uni in February. The
question seemed to be way over most of our heads, I'm assuming the lecturer gave us the question to make us do some research over the weekend.
So because we are using NaOH as the base it is only strong enough to attack the outside part of the chain which is the weakest part of the molecule
and leaving the ethyl group in tact. So if we wanted to get at the ethyl group for arguments sake we would need to use a stronger base?
Can I ask what programe you made that molecule drawing on?
[Edited on 16-1-2015 by Muzz1969]
[Edited on 17-1-2015 by Muzz1969]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
What makes those hydrogens on the ethylene bridge so difficult to remove is the fact that it's a dicarboxylic acid. The two hydrogens on the carboxyl
groups are much more acidic than the others and are always going to be the first to go. Keep in mind that each additional deprotonation is more
difficult than the previous. Even the second carboxyl group is harder to deprotonate than the first. By the time the alpha-hydrogens are next in line,
the molecule's already lost two protons.
If you really needed to remove an alpha-hydrogen for some reason, say to add an alkyl group or something, one way to do it is to just convert the
whole thing into a diester first. The alpha-hydrogens would then become the most acidic on the molecule and a lot easier to remove, as a much more
stable enolate can now form. Once you've done what ever it is you needed to do, you could then hydrolyze it back to a carboxylic acid.
ChemDraw Pro.
[Edited on 17-1-2015 by Darkstar]
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Right that a bit over my head but I do understand what you are getting at and has given me a clearer picture. When talking about the level of hydrogen
bonds are they sequentially classified alpha, beta etc? Moving from the closest bond to the less stable on the outside of the molecule....
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Marvin Sketch is a free molecular modelling soft-ware suite. I highly recomend it Darkstar.
|
|
|
Muzz1969
Harmless

Posts: 37
Registered: 19-11-2014
Member Is Offline
Mood: No Mood
|
|
Thanks guys for all your help
|
|
|