Anisaldehyde (4-Methoxybenzaldehyde [2])
A freshly prepared and stirred solution of 30 mL concentrated sulfuric acid in 150 mL water was allowed to cool down to 30° C, and anise oil (9.8 g)
was added. A total of 25 g sodium bichromate was then added, at such a rate that the reaction temperature remained between 35-40° C. The reaction
mixture was extracted four times with toluene (75 mL each), and the combined organic phases were washed twice with 5 % NaOH (100 mL each), and once
with water (100 mL). The organic phase was evaporated to about 20 mL, and anisaldehyde was then isolated as its bisulfite adduct. The yellow
precipitate was washed with an EtOH/ether (1:1) mixture until the precipitate's color turned white (that is, similar to the bisulfite adduct generated
from commercially available anisaldehyde). Setting the anisaldehyde free resulted in 4.9 g of a yellow oil with a pleasant odor. The mass spectrum was
in agreement with an authentic sample. Anisaldehyde was the main product (95 % by GC/MS), but several minor impurities (not further identified in this
report) were noted. |



 .
. thanks for the help!
thanks for the help!













 .
.





















 and have other
projects to attend to (TEMPO derivatives, etc).
and have other
projects to attend to (TEMPO derivatives, etc). I would be delighted to obtain ~50gr at a reasonable price.. I'm sure we could work out a compassion
I would be delighted to obtain ~50gr at a reasonable price.. I'm sure we could work out a compassion 



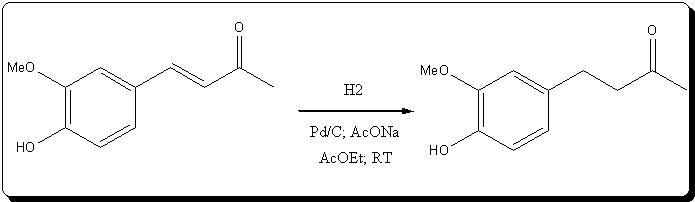
 et Ether, 1.61g (8.30
mmol) of practically colorless zingerone was obtained, giving a 74.11% yield from the unsaturated ketone, and
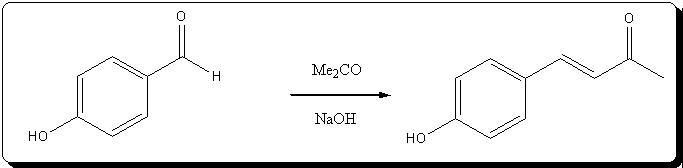
59.18% from vanillin.
et Ether, 1.61g (8.30
mmol) of practically colorless zingerone was obtained, giving a 74.11% yield from the unsaturated ketone, and
59.18% from vanillin.

 et ether).
et ether).













 et ether) indicated saturated ketone (major stain), a little unsat
ketone, and two other spots (dimers).
et ether) indicated saturated ketone (major stain), a little unsat
ketone, and two other spots (dimers). et ether as an eluant proved to give a superior seperation than
with the eluant used during the Zn reduction. I am unsure of which side-products are formed during the reduction, but I'm pretty sure Zn/AcOH can't be
used as a preparative procedure for phenolic butanones..
et ether as an eluant proved to give a superior seperation than
with the eluant used during the Zn reduction. I am unsure of which side-products are formed during the reduction, but I'm pretty sure Zn/AcOH can't be
used as a preparative procedure for phenolic butanones..



 Pretty strangely, the nice crystals smell much less than the crude powder, before recrystallization.
Pretty strangely, the nice crystals smell much less than the crude powder, before recrystallization.






