The ‘convential’ opioids (some like to refer to these as opiates as a loose definition) are based on naturally extracted morphine. Most of the
common opioid painkillers are just small tweaks to the morphine molecule, but oxycodone is a bit further away, having a ketone, saturated alkene,
methyl ether (same as in codeine) and an additional hydroxy group.
Actually I found that studying the molecular structures of biochemical and pharmaceutical compounds (legal and illicit, the distinction can get
blurred very quickly but the chemistry doesn’t) is what really got me interested in organic chemistry after the basic stuff in school. Never really
viewed theoretical chemistry as a taboo in any way, even with stuff like explosives and highly poisonous chemicals - it’s when that information is
applied in practice that the problems arise. It’s not like we’re showing people how to make them or anything, or even providing information that
wouldn’t be found with a quick search on Google, I guess that’s the main reason why I feel quite relaxed discussing any chemistry in which I’m
at least somewhat knowledgeable, even if I haven’t or have no intention of carrying it out in practice.
I suck at explaining how these sorts of things work or how to use it, it’s just one of those things you need to have a play around with to get
familiar with, plus it’s not like you have to pay for anything if it’s useless from the start. I’ve been tempted to get some Molymod kits to be
honest, but downloading an app that does the same thing and has a lot more functionality kinda replaced that. Still would be cool to have a few
molecules sitting around the lab though.
Oh, I just grabbed the first two examples I thought of, where NH2 is an electron donating group and both OH and COOH are electron withdrawing. Charts
are good for the basic rules but having the isosurface there really paints a better picture of what the electrons are doing. I don’t think you can
readily synthesise one from the other, not in just a couple of steps anyway - paracetamol is usually made by the n-acetylisation of 4-aminophenol,
whereas aspirin is made by o-acetylating salicylic acid, both with acetic anhydride.
|



 . But considering I got no where and you quickly delivered.......
. But considering I got no where and you quickly delivered.......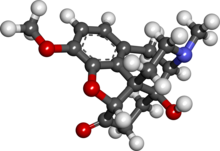






 )
)


