Despite my disinterest in discussing the chemistry directly, I will throw out a quip about the thread evolution. I can't speak on the totality of your
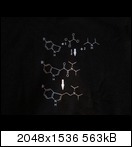
specific compounds of interest, but some 5-halogenated simple tryptamines, such as are being discussed (contrast with ergolines) have been done by
others than Shulgin. As renouned as the man was, he did not work in a vacuum. See Benington, Kochanowska, Bower, Nichols, etc. Bit of a niche market,
in an academic sense. Quote: Originally posted by CuReUS  | | Tikhal,but couldn't find it,there was only 5-OH and 5-OMe.but do you think the long chain trips are any good.? DMT is visual,DET is auditory and DPT
is sensory,but others ? |
Good? There is no such thing in pharmacology. Please be specific with the efficacy
you refer to. Do you mean to ask if they are psychoactive? Any more specificity than a "yes" or "no" to any degree of confidence (i.e.
probably/unlikely) is guesswork, as serotonin has a plethora of discovered receptors of various types, and almost certainly more to be discovered,
much less specifics of the neural pathways they are involved with. There is plenty of evidence out there that some psychoactive trypamine effects are
pharmacodynamically modulated by more than one serotonin receptor, and some psychoactive effects have been correlated to these (loosely), and this is
ignoring transporters and metabolic enzymes for simplicity (they have downstream pharmacodynamic effects). Trying to guess psychoactive effects
without some serious SAR and experimental data (or just the right empirically derived receptor model) per various receptors, in any real detail, is
extremely troublesome once modifications begin. It also doesn't take into account the complexities of potential toxicity, which may outweigh any
desired efficacy.
Often in pharmacology, extension of the alkyl substituents causes decreased affinity and subsequent diminishment of efficacy. In the crudest of
senses, this may be assumed the case for (possibly erroneously) through comparing methylbutyltryptamine doses with the dimethyl versions, as well as
ethyl variants, taking into account differences in bioavailability per route of administration and normalizing appropriately. Consider also the alpha
alkyls, for further elucidation of sterics of various receptors. Psychoactive distinctions get interesting as you're one step removed from the
neuropharmacology, and have to get into surrogate measures on top of the usual animal models in many instances. Biol Psychiatry. 1983
Jul;18(7):829-36. might be a good example. |


 .
.











 )
)






 .Maybe DMDO could be used
as well
.Maybe DMDO could be used
as well




 .
.