| Pages:
1
2 |
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
5-Bromoindole preparation
Hello!
I would like to prepare 5-Bromoindole, and the usual procedure from Indole ive found, in means of making the sodium 2-indoline sulfonate, and then
protecting of the nitrogen with acetic anhydride isnt so nice, as huge amounts of Ac2O are needed, and its hard to get for hobbyists.
I would like to know if anyone can suggest another Protecting group, to deactivate the pyrrole ring, which is also removable with base.
I would further brominate the sodium 1-acetyl indoline-2-sulfonate with bromine, then cleave the protecting groups on 1- and 2- with alkali
hydroxides, to get 5-bromoindole.
I have much Indole, so i would like to do this route very much if I just find another suitable protecting group for the N-atom.
Ive also looked into making this compound from 5-bromo-2-nitrobenzaldehyde, via henry reaction with nitromethane, then reduction of the nitrostyrene
to an aldehyde and the aromatic nitro group to an amine, with Fe dust and Acetic Acid, which would also result in 5-bromoindole.
But here I cant think of any easy synthesis of the benzaldehyde derivative.
Can somebody help me please?
Both these routes are already close to the price of 5-bromoindole, from which I dont need much of, but i really would like to perform some experiments
with it. Simply buying it isnt what i want, and yes, Ac2O is nothing I can get easily, but 5-bromoindole is.
My references are:
5-bromoindole synthesis, erowid
4-acetoxyindol synthesis, lambdasyn
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Might be able to form from 4-bromoaniline phenylhydrazone.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Thank you for replying!
I thought about the Fischer-Indole synthesis as well, hey, i´ve even printed it on a shirt  . .
But its such a long troublesome way, compared to the Speeter-Anthony (which I also printed on a shirt).
I would like to prepare some Analogues of some marine Tryptamines.
Well, I think I am just buying the bromoindole then if no ones else has a good idea...
Its not that I want to produce huge amounts, im just curious. A few grams would suffice and its not that expensive.
The synthesis via 5-bromo-2-nitrobenzaldehyde looks appealing, but i neither cant find something, nor think of any feasible route to it.
Could somebody help on this part maybe?
Fischer-Indole synthesis:

Speeter-Anthony synthesis
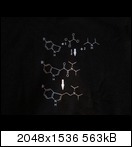
[Edited on 20-3-2015 by karlos³]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
is 5-bromo-dmt your final product?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
The synthesis via 5-bromo-2-nitrobenzaldehyde looks appealing, but i neither cant find something, nor think of any feasible route to it.
|
a quick google search should give many results
you could try nitrating 3-bromobenzaldehyde using H2SO4/HNO3 at room temp for 4 hours to get the
5-bromo-2-nitrobenzaldehyde
http://khimiya.org/pdfs/052-065%20Pages%20from%20Khimiya%200... see page 7
synthesis of 3-bromobenzaldehyde
https://bbzfrankie.wordpress.com/2013/09/10/how-to-prepare-3...
http://www.google.com/patents/US4945186
[Edited on 20-3-2015 by CuReUS]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Thank you CuReUS, stupid me forgot that theres a Orgsyn preparation for 3-halogenation of benzaldehydes out there, which was the main problem I
couldnt solve in the preparation of the 5-bromo-2-nitrobenzaldehyde, or otherwise spoken I havent thought of the 3-position for the halogen is after
nitration the 5- position correctly named.
3-Halogenation of Acetophenone/Benzaldehyde
I also stumbled over the Tyrian Purple Syntheses, but forgot that they also prepared a 5,5-dibromo-indigo derivative of Tyrian Purple
(6,6-dibromo-indigo).
Thank you, that was helpful from you puzzling all the pieces together, I was so focused on other parts of the whole thing, I didnt thought of this.
You´ve helped very much! 
@waffles SS: No, not the 5-Bromo-N,N-Dimethyltryptamine, I am interested in the longer N,N alkylchains, the Diethyl, Dipropyl, and Diisopropyl
Derivatives.
They look appealing to me, as Dr. Shulgin never made any halogenated Tryptamine as far as I know, but so many halogenated Phenylethylamines.
Should be worth a shot, Speeter-Anthony Tryptamine Synthesis, then reduction of the Dialkylglyoxylamides with Vitride als LiAlH4 substitute, fast and
feasible.
As I said, they look appealing and are easy to make.
Also, 5-Bromo-N,N-Dimethyltryptamine is an active one, but gets possibly destroyed by MAO, thats why I would like to make the longer Dialkylamines
than Dimethyl.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Try below:

5-bromo indole from indole
1: sodium hydrogensulfite / ethanol; water / 20 h / 20 °C
2: 2.5 h / 70 - 90 °C
3: water; bromine / 2 h / 0 - 20 °C
Antifungal agents. Part 4: Synthesis and antifungal activities of novel indole[1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic
fungi in vitro
http://www.sciencedirect.com/science/article/pii/S0223523410007622
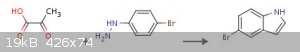
5-bromo indole from 4-bromo phenyl hydrazine + pyruvic acid
(With phosphorus pentachloride; zinc(II) chloride )
(Fischer indole synthesis)
A new and efficient one-pot synthesis of indoles
http://www.sciencedirect.com/science/article/pii/S0040403907024550
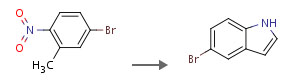
5-bromo indole from 2-nitro 5-bromo toluene
1: pyrrolidine / dimethylformamide / 110 °C
2: 47 percent / Zn / acetic acid / 2 h / 85 °C
Metal-halogen exchange of bromoindoles. A route to substituted indoles
http://pubs.acs.org/doi/abs/10.1021/jo00376a010
[Edited on 20-3-2015 by Waffles SS]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Dunno. If you have Indole, and only wish to form 5-Bromo-Indole, Erowid seems to have a decent procedure.
My Ninth edition of the Merck Index, suggests that 4-Bromophenylhydrazine, prepared from 4-Bromo-aniline, is a useful intermediate for producing
5-Bromo-Indole Acetic acid, via the Fischer.
Big Pharm, may be using the Fischer, as a route to Sumatriptan. As I recall, a Diazonium Salt, is reacted with Dihydrofuran to yield a
phenylhydrazone. Which is then cyclized to form a Tryptophol. At which point, most of the heavy lifting has already been accomplished. You have a
ring substituted tryptamine skeleton, now manipulate as required.
http://en.wikipedia.org/wiki/Sumatriptan
Not the exact sequence I'm recollecting, but interesting.
[Edited on 20-3-2015 by zed]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
No, not the 5-Bromo-N,N-Dimethyltryptamine, I am interested in the longer N,N alkylchains, the Diethyl, Dipropyl, and Diisopropyl Derivatives.
They look appealing to me, as Dr. Shulgin never made any halogenated Tryptamine as far as I know |
yes,after I my last post,I immediately searched for 5 bromo tryptamine in Tikhal,but couldn't find it,there was only 5-OH and 5-OMe.but do you think
the long chain trips are any good.?
DMT is visual,DET is auditory and DPT is sensory,but others ?
| Quote: | | Should be worth a shot, Speeter-Anthony Tryptamine Synthesis, then reduction of the Dialkylglyoxylamides with Vitride als LiAlH4 substitute, fast and
feasible. |
could Zn/Hg or Al/Hg in conc HCl be used instead of LiAlH4 ?also be careful,you don't want the pictet spengler to happen
also TCCA in presence of 50% aq H2SO4 as catalyst could chlorinate the ring.even NBS might work ?
Quote: Originally posted by zed  |
Big Pharm, may be using the Fischer, as a route to Sumatriptan. As I recall, a Diazonium Salt, is reacted with Dihydrofuran to yield a
phenylhydrazone. Which is then cyclized to form a Tryptophol |
tryptophol can be made by fermentation
http://www.sciencemadness.org/talk/viewthread.php?tid=39224#...
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Despite my disinterest in discussing the chemistry directly, I will throw out a quip about the thread evolution. I can't speak on the totality of your
specific compounds of interest, but some 5-halogenated simple tryptamines, such as are being discussed (contrast with ergolines) have been done by
others than Shulgin. As renouned as the man was, he did not work in a vacuum. See Benington, Kochanowska, Bower, Nichols, etc. Bit of a niche market,
in an academic sense. Quote: Originally posted by CuReUS  | | Tikhal,but couldn't find it,there was only 5-OH and 5-OMe.but do you think the long chain trips are any good.? DMT is visual,DET is auditory and DPT
is sensory,but others ? |
Good? There is no such thing in pharmacology. Please be specific with the efficacy
you refer to. Do you mean to ask if they are psychoactive? Any more specificity than a "yes" or "no" to any degree of confidence (i.e.
probably/unlikely) is guesswork, as serotonin has a plethora of discovered receptors of various types, and almost certainly more to be discovered,
much less specifics of the neural pathways they are involved with. There is plenty of evidence out there that some psychoactive trypamine effects are
pharmacodynamically modulated by more than one serotonin receptor, and some psychoactive effects have been correlated to these (loosely), and this is
ignoring transporters and metabolic enzymes for simplicity (they have downstream pharmacodynamic effects). Trying to guess psychoactive effects
without some serious SAR and experimental data (or just the right empirically derived receptor model) per various receptors, in any real detail, is
extremely troublesome once modifications begin. It also doesn't take into account the complexities of potential toxicity, which may outweigh any
desired efficacy.
Often in pharmacology, extension of the alkyl substituents causes decreased affinity and subsequent diminishment of efficacy. In the crudest of
senses, this may be assumed the case for (possibly erroneously) through comparing methylbutyltryptamine doses with the dimethyl versions, as well as
ethyl variants, taking into account differences in bioavailability per route of administration and normalizing appropriately. Consider also the alpha
alkyls, for further elucidation of sterics of various receptors. Psychoactive distinctions get interesting as you're one step removed from the
neuropharmacology, and have to get into surrogate measures on top of the usual animal models in many instances. Biol Psychiatry. 1983
Jul;18(7):829-36. might be a good example.
|
|
|
Scr0t
Hazard to Others
  
Posts: 118
Registered: 14-1-2012
Location: Europe
Member Is Offline
Mood: Desiccated
|
|
DET is definitely visual and sensory, it's DiPT that primarily targets auditory perception.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  | Despite my disinterest in discussing the chemistry directly, I will throw out a quip about the thread evolution. I can't speak on the totality of your
specific compounds of interest, but some 5-halogenated simple tryptamines, such as are being discussed (contrast with ergolines) have been done by
others than Shulgin. As renouned as the man was, he did not work in a vacuum. See Benington, Kochanowska, Bower, Nichols, etc. Bit of a niche market,
in an academic sense. Quote: Originally posted by CuReUS  | | Tikhal,but couldn't find it,there was only 5-OH and 5-OMe.but do you think the long chain trips are any good.? DMT is visual,DET is auditory and DPT
is sensory,but others ? |
Good? There is no such thing in pharmacology. Please be specific with the efficacy
you refer to. Do you mean to ask if they are psychoactive? Any more specificity than a "yes" or "no" to any degree of confidence (i.e.
probably/unlikely) is guesswork, as serotonin has a plethora of discovered receptors of various types, and almost certainly more to be discovered,
much less specifics of the neural pathways they are involved with. There is plenty of evidence out there that some psychoactive trypamine effects are
pharmacodynamically modulated by more than one serotonin receptor, and some psychoactive effects have been correlated to these (loosely), and this is
ignoring transporters and metabolic enzymes for simplicity (they have downstream pharmacodynamic effects). Trying to guess psychoactive effects
without some serious SAR and experimental data (or just the right empirically derived receptor model) per various receptors, in any real detail, is
extremely troublesome once modifications begin. It also doesn't take into account the complexities of potential toxicity, which may outweigh any
desired efficacy.
Often in pharmacology, extension of the alkyl substituents causes decreased affinity and subsequent diminishment of efficacy. In the crudest of
senses, this may be assumed the case for (possibly erroneously) through comparing methylbutyltryptamine doses with the dimethyl versions, as well as
ethyl variants, taking into account differences in bioavailability per route of administration and normalizing appropriately. Consider also the alpha
alkyls, for further elucidation of sterics of various receptors. Psychoactive distinctions get interesting as you're one step removed from the
neuropharmacology, and have to get into surrogate measures on top of the usual animal models in many instances. Biol Psychiatry. 1983
Jul;18(7):829-36. might be a good example. |
Holy shit, a thread 10 posts long with 2 isolated sentences on subjective bioactivity...
Hell, that kind of subjective language is quite common from biochemists/pharmacologists/medicinal chemists when they aren't talking in a formal
setting. It makes it easier to give a subjective opinion of efficacy without having to list each metabolic step or inhibition/variation that could
occur. The last part of the sentence is pretty definitive of "good" in context.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by CuReUS  |
could Zn/Hg or Al/Hg in conc HCl be used instead of LiAlH4 ?also be careful,you don't want the pictet spengler to happen
|
Well I havent heard of any way to reduce the Indolglyoxylamide with any other Reducing Agent, you can maybe reduce the ketone this way, but not the
amide carboxylic group.
I think besides Vitride/Red-Al which I want to use, theres only LiAlH4, probably some Diborane reduction Agent, and something else, I suspect it was
Zinc Borohydride which reduces amides to amines?
But i really prefer Red-Al, thats a handy reagent, much more harmless then LiAlH4 to work with, almost all the same applications that it can be used
for, but much less pyrophoric and so soluble, no suspensions, i like it.
| Quote: | also TCCA in presence of 50% aq H2SO4 as catalyst could chlorinate the ring.even NBS might work ?
|
Ive read a paper about using NBS on tryptophol and indole acetic acid, and they dont produce the 5-bromo derivative, i think it was the double bond on
the pyrrole ring which directs them to position 3- or 2, cannot remember, but sadly nothing what i want.
But if NBS could be substitute for elemental bromine on Sodium Indoline-2-Sulfonate, directly, that would be a very comfy and harmless synthesis to
do, very appealing.
@Waffles SS:
The first reaction directly from Indole you mentioned, using sodium hydrogensulfite, sounds similiar to the route i found on erowid, but without
adding an acetyl-group to the nitrogen before bromination.
That sounds pretty interesting, thats what I was looking for, i am going to ask for the paper in the references thread to get a look at the
conditions, but that sounds very compromising and short.
Maybe the sulfonate is just bulky enough to inactivate the whole pyrrole ring, and allows direct bromination of the aromatic ring on position 5-.
That would be very nice.
Thank you!
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mesa  |
Holy shit, a thread 10 posts long with 2 isolated sentences on subjective bioactivity...
Hell, that kind of subjective language is quite common from biochemists/pharmacologists/medicinal chemists when they aren't talking in a formal
setting. It makes it easier to give a subjective opinion of efficacy without having to list each metabolic step or inhibition/variation that could
occur. The last part of the sentence is pretty definitive of "good" in context. |
People in the field use all kinds of language informally, especially after 5:00pm, but that isn't relevant to clarifying intended efficacy or the
forum's semi-professional demeanor. Anyone in drug discovery and development has a target receptor in mind for a project.
A drug's benefit derives directly from intended use, which is very subjective in a non-medicinal setting. Is longer half-life (functional or chemical)
important? Some psychoactive drugs last too long for the general use, recreational or otherwise, despite a duration of time between maintenance doses
generally being favorable in medicine for patient compliance. Some psychoactives are dysphoric in nature, or some exhibit partial agonism at some
receptors (particularly the 5-fluoro tryptamine, if anyone looks into the authors I mentioned, who synthesize and characterize such compounds).
Clearly this may be undesired, despite my finding it interesting, so clarification is paramount.
Giving a subjective opinion of efficacy without a target or empirical data, even a bioassay, is worthless in science, especially when
efficacy is undefined. Ignoring binding promiscuity of the parent compound, no metabolites, is just having any expected psychoactive effect "good?" Is
dosage and cardiac toxicity of the parent compound (ignoring metabolites) an overriding concern? Are you aiming to try and replicate a specific type
of 'trip' (type of hallucinationatory, euphoric, entactogenic, etc.) by replicating a pharmacophore for a specific receptor? Do you want to try to
bias the functional selectivity thereof to see how that alters an experience? Do you want to try and reduce MAO metabolism, because this is obviously
important in determining psychoactivity?
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by Mesa  | Holy shit, a thread 10 posts long with 2 isolated sentences on subjective bioactivity...
Hell, that kind of subjective language is quite common from biochemists/pharmacologists/medicinal chemists when they aren't talking in a formal
setting. It makes it easier to give a subjective opinion of efficacy without having to list each metabolic step or inhibition/variation that could
occur. The last part of the sentence is pretty definitive of "good" in context. |
"DMT is visual,DET is auditory and DPT is sensory,but others ?" doesn't do anything to define what the hell "good" means in context.
[Edited on 3-21-2015 by Etaoin Shrdlu]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Karlos, have you checked any primary literature from other authors? Clearly you have done your research, so I don't mean to imply you have done none
at all, but I am curious what you have looked at other than lambdasyn, erowid, and TIHKaL. Some authors have made various derivatives, often starting
from a sourced substituted indole (not of as much use to you), but this was not always commercially available according to one publication.
[Edited on 21-3-2015 by Chemosynthesis]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Well ive seen the report somebody sent to Halmilton Morris from Vice on 5-Bromo-DMT which personal description of the effects of smoked
5-bromo-dimethyltryptamine, not the perfectly serious source, but it got me interested in what the other 5-bromodialkyltryptamines might do in humans.
I also know about some 5- or 6- Fluorotryptamines, but a fluorine atom is so much different in course of biological action, its not bulky enough to
have the activity im interested in.
I even read a paper about brominated Psilocin analogues, but as far as I remember it doesnt mentioned any other longer dialkylamine groups than
dimethyl.
Im just curious, even if my compounds in mind show they are inactive in psychoactive means, it doesnt matter much.
I simply want to synthesise those marine alkaloid analogues as pet project.
Also, 5-Bromoindole is available commercially, and it is comparably cheap, but not compared to the price of indole.
around 30 euros isnt what I want to pay for 5 grams of the substituted Indole, if for the same price im able to get a hundred grams of normal Indole,
so, for the matter of making three differenct compounds in amounts which serve well enough for human testing, compared to the unsubstited
dialkyltryptamines.
I am in the know of the activity of DET, DPT and the weird acoustic DiPT, i would really like to make at least a few gramms of brominated
indoledialkylglyoxylamide of every of these three dialkylamine derivates, to compare their effect to the standard unsubstituted dialkyltryptamine.
No other analogue than the diethyl- dipropyl- and diisopropylamine of these is planned.
Maybe later then a 5-Iodo analogue of the most promising of them, there are some marine alkaloids called plakohypaphorines, which are halogenated
quarternary trimethyltryptophane salts.
Iodine might be able to produce some interessting biological activity
(Just think about, 5-Bromo-Diisopropyltryptamine might be able to let you hear the waves breaking... just some sort of a joke i tell when asked about
the intention of this unusual project ) )
[Edited on 22-3-2015 by karlos³]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
I also know about some 5- or 6- Fluorotryptamines, but a fluorine atom is so much different in course of biological action, its not bulky enough to
have the activity im interested in. |
Florine does usually substitute better for hydrogen than other halogens, though it is very useful to demonstrate what kind of modifications shift
serotonin subtype affinity profiles, and possibly for synthesis schemes. Look into an author by the name of Benington I mentioned above. I listed him
in addition to Bower and Nichols for a reason. The latter assist with the 5-fluoro pharmacophore models in serotonin receptor subtypes, and sterics
such as with chlorine. The former and his group synthesized the 5-chlorotryptamine, a bioisostere rather than classical/chemical one, back in 1960.
His work should be interesting to you in particular due to the following:
| Quote: | | Ive also looked into making this compound from 5-bromo-2-nitrobenzaldehyde, via henry reaction with nitromethane, then reduction of the nitrostyrene
to an aldehyde and the aromatic nitro group to an amine, with Fe dust and Acetic Acid, which would also result in 5-bromoindole
|
You will find his data on the analogous chloro derivative synthesis interesting, I am sure. His reductive cyclization was a little different, but the
results (or lackthereof) are worth looking at.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yeah Im looking into it right now... I guess their use of Pd/C was the cause for the dehalogenation during the cyclisation.
I dont think this will happen when using Fe in acetic acid, or better, hope it.
Especially the sentences | Quote: | When the reductive cyclization of XXVII (5-chloro-2-nitrophenylnitrostyrene) was attempted in accordance with the method described
by Huebner, indole was obtained instead of 5-
chloroindole. This result is not surprising in light
of the findings of Strel'tsova and Zelin~kii, who
have demonstrated that hydrogenolysis of the
halo group occurs simultanieously with reduction
of the nitro group when either 2- or 4-chloronitrobenzene
is treated with hydrogen in the presence of
a noble-metal catalyst (palladium or platinum).
Since 5-chloroindole became commercially available
during this study, this was used in the synthesis
of the tryptamine VIII. |
sound very discouraging.
http://bitnest.ca/external.php?id=%257DbxUgZ%255BC%2540Z%2504uzx%250DTWYQ
My lack of Ac2O is disturbing, they use so much to for the acetylation of 2- Sodium Indoline Sulfonate, and i cant find another decent way to
deactivate the pyrrole ring for aromatic bromination,
Fischer indole on 4-Bromophenylhydrazone with dihydrofuran sounds better, despite the extra work, because making a tryptamine from tryptophol is a
very rewarding experience, when doing it via the tosylate ester.
[Edited on 22-3-2015 by karlos³]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
If it works, I would find that very interesting, despite my expressed disinterest before.
To add to the discouragement, just in case you're not aware (sorry to be pedantic if you are), Nichols used the Hemetsberger–Knittel indole
synthesis with methyl azidoacetate for several fluoro derivatives, and I am assuming you would like to avoid this (I would). While impressive, I am
not sure he would have gone through this if not necessary.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
Well I havent heard of any way to reduce the Indolglyoxylamide with any other Reducing Agent, you can maybe reduce the ketone this way, but not the
amide carboxylic group. |
actually I read this in a text book where they reduced the amide with Zn/HCl to get mescaline.But surprisingly I couldn't find a reference anywhere.
| Quote: | | But if NBS could be substitute for elemental bromine on Sodium Indoline-2-Sulfonate, directly, that would be a very comfy and harmless synthesis to
do, very appealing. |
I think NBS can be substituted for elemental bromine in all reactions.The only difference between them is that for Br2,you use a drip
funnel,whereas NBS slowly releases Br2 in the reaction medium itself.That's why many labs nowadays use TCCA or hypochlorite instead of
Cl2 gas for their chlorination reactions.
I had another reaction in mind.Suppose you started of with o-toulidine.reacting this with Br2 in CS2 or any other non polar
solvent should give you the 5-bromo-toulidine easily,due to the activating nature of NH2
then you could oxidise the NH2 to NO2 using H2O2 and do the resseirt indole synthesis
http://en.wikipedia.org/wiki/Reissert_indole_synthesis
[Edited on 22-3-2015 by CuReUS]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
actually I read this in a text book where they reduced the amide with Zn/HCl to get mescaline.But surprisingly I couldn't find a reference anywhere.
|
Even good texts have errors. I have caught problems in medicinal chemistry books that mischaracterized
citations, generalizing inappropriately to substrates and complained to editors. No fix last I checked. You would think correcting a factual
inaccuracy would be on the publisher's "to-do" list since a new edition with corrections reduces the appeal of the secondary sales textbook market,
from which a publisher makes no income.
| Quote: | | I think NBS can be substituted for elemental bromine in all reactions. |
Not all. It offers greater selectivity, similar to low concentrations of bromine, without this restriction on concentration being as important. You
use it to avoid dihalogenation of an alkene in lieu of allylic bromination, for example.
| Quote: | I had another reaction in mind.Suppose you started of with o-toulidine.reacting this with Br2 in CS2 or any other non polar
solvent should give you the 5-bromo-toulidine easily,due to the activating nature of NH2
then you could oxidise the NH2 to NO2 using H2O2 and do the resseirt indole synthesis
http://en.wikipedia.org/wiki/Reissert_indole_synthesis
|
Doubtful. Giving you the benefit of the doubt on cleanliness of a peroxide oxidation of the given substrate, this doesn't address the cyclization
issue I brought up. Read the source material, not the Wikipedia summary!
Org. Synth. 1963, 43, 40
A
Karlos' interest in iron rather than zinc should allow gentler conditions, though I am a bit skeptical they will be gentle enough. Zinc is moving in
the opposite direction of the electrochemical activity series. Fischer and indole bromination are still top contenders.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
| Quote: | | Fischer and indole bromination are still top contenders. |
I am currently mostly interested in the Fischer to form 5-Bromo-Tryptophol, because ive read somewhere in the old hive posts, that instead of
dihydrofuran other compounds can be used in the condensation with the Phenylhydrazine, to form Tryptophol. 2,3-Dihydrofuran isnt that easily available
to me, so what could do the trick instead here? Is there any reagent thats more common? I stumbled over some other synthons, like Dihydropyran, but
there are others, right?
4-Bromophenylhydrazine preparation from aniline should be easy, and compared to synthesising an dialkylaminobutanal-dialkyl acetal also a satisfactory
deed.
From Tryptophol on, i know the reactions first hand, and even if the aminolysis of the ester uses much dialkylamine and takes days, its a very nice
and clean synthesis.
Nothing fast like Speeter Anthony, but nonetheless also a very convenient reaction.
And to say it at least once, im very excited about all of your ideas offered, I really appreciate that, thank you all for chiming in into the
discussion! 
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, no route is especially easy. I suggested the Fischer because it avoids harsh reduction conditions. Just off hand, I don't know how well
5-Bromoindoles hold up to hydride reductions, and someone has already cited an example of dehalogenation via Pt/H2.
The formation of trypamines via the Grignard reaction works quite well, and is fairly straightforward, but once again...I do not know if Bromine at
the 5-position can stand up to the reaction conditions.
The synthesis via the use of Dialkylamino- butyraldehyde looks OK, but you usually have to make the aldehyde, which is no small task. Likewise, you
might have to make your own 2,3-dihydrofuran.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
LiAlH4 Reduction leaves the Bromine atom untouched, and i think Red-Al will too.
Ive worked with indole grignards, reacted them with acid chlorides, and I personally dont like those reactions (even if i like grignard reactions very
much, not those involving indole).
Even if I had 5-Bromoindole on hand, i wouldnt use it to make tryptophol or an dialkyltryptamine via grignard, because all the synthons for those are
very toxic, like ethylene oxide for tryptophol, or those nasty mustard gas precursors for dialkyltryptamines.
|
|
|
| Pages:
1
2 |
|