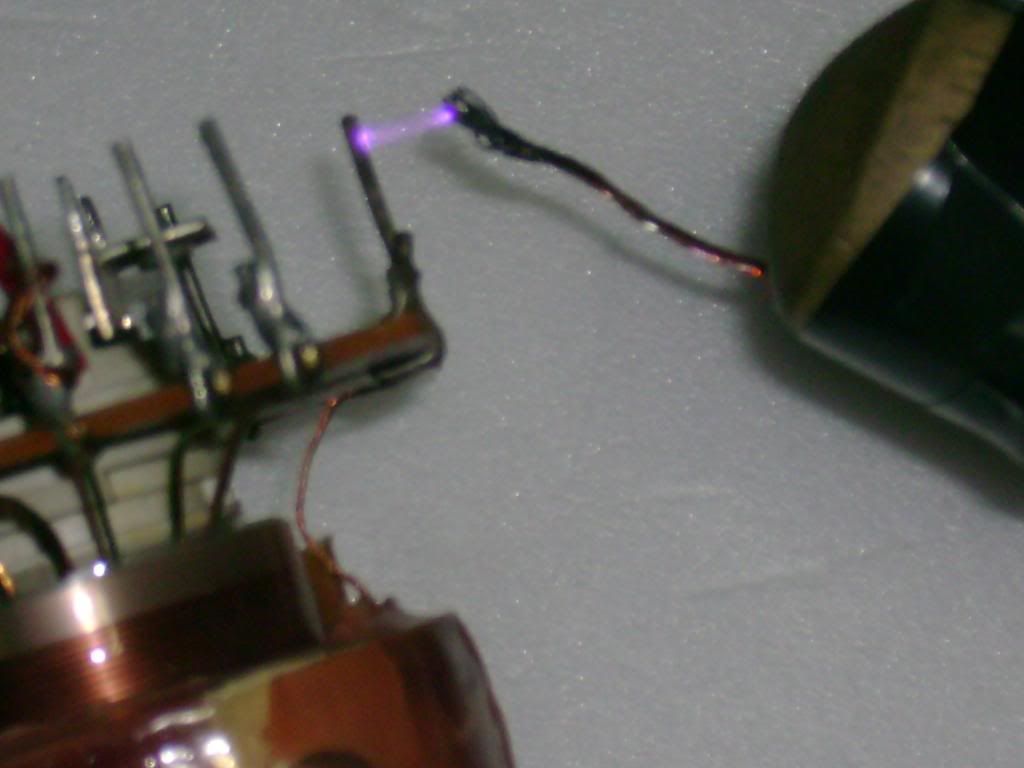
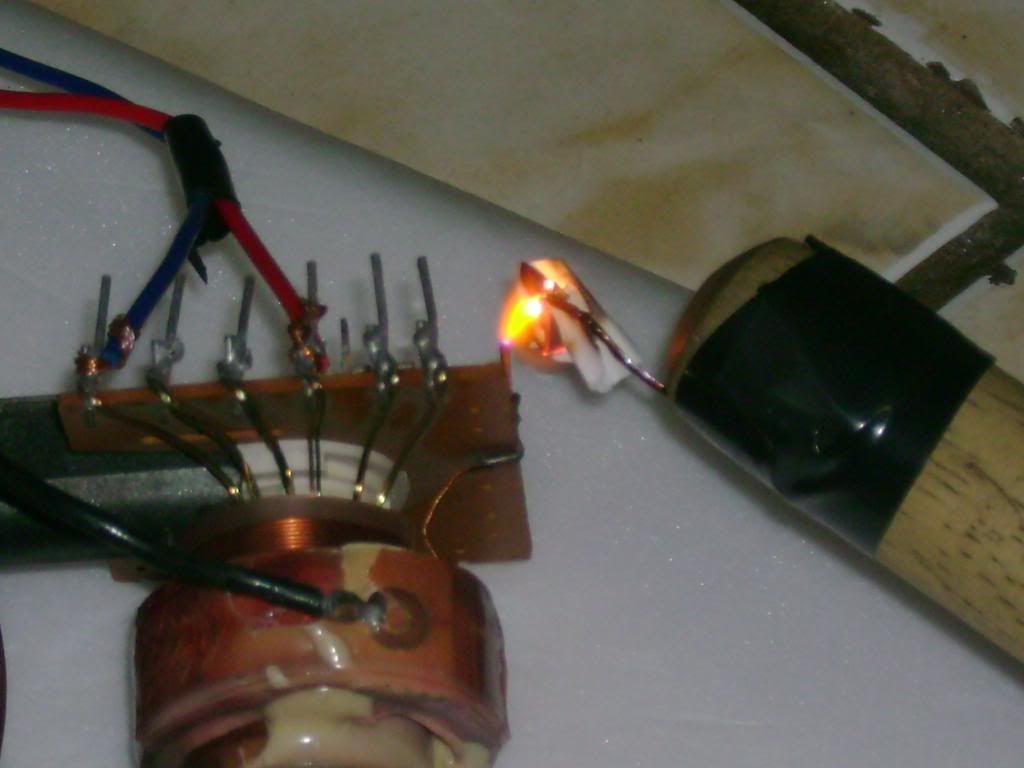
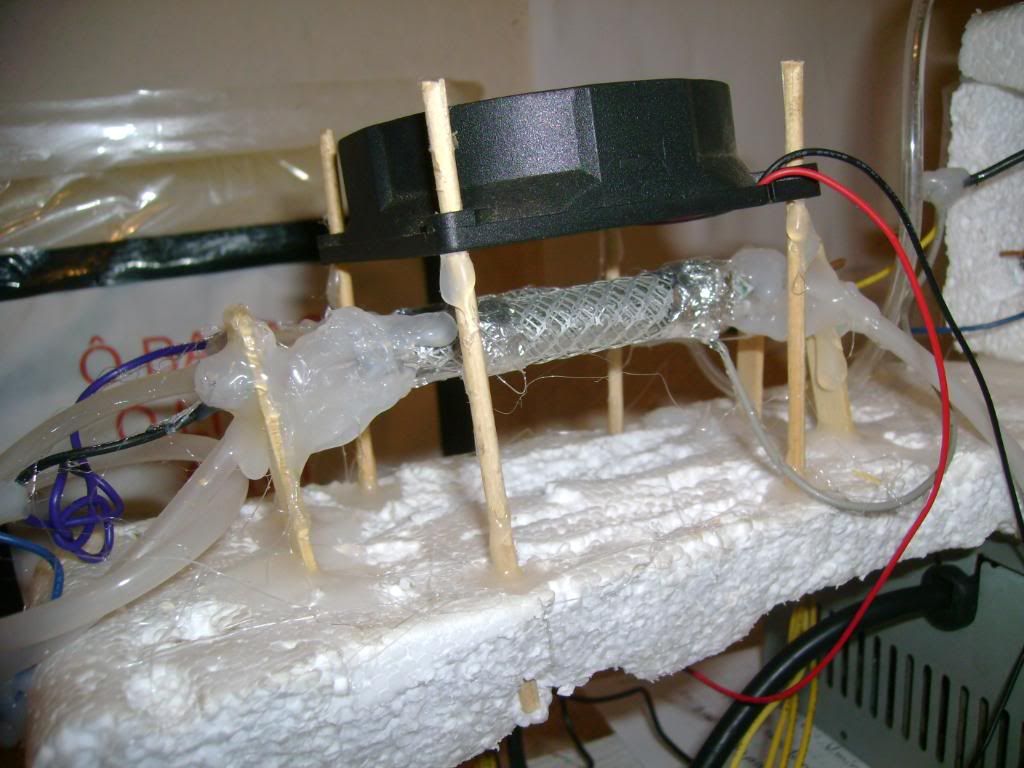
Small quantities of ozone are produced when oxygen and air are subjected to an electrical discharge and it is, therefore, found in the neighbourhood
of working electrical machines. Probably a small quantity of atomic oxygen is initially produced; most of this recombines quickly to give oxygen,
O2, but a few atoms react to form ozone:
O2 + O --> O3
The ozone molecules also decompose by reaction with atomic oxygen, so that the actual concentration of ozone is small.
Ozone is formed in certain chemical reactions, including the action of fluorine on water [...] and the thermal decomposition of iodic(VII) (periodic)
acid. It is also formed when dilute (about 1 M) sulphuric acid is electrolysed at high current density; at low temperatures the oxygen evolved at the
anode can contain as much as 30% ozone.
Ozone is normally produced by the use of a silent electrical discharge and a number of ozonisers have been produced. Brodie's apparatus is shown in
outline in [file attachment].
Using a potential of approximately 20000 V the ozonised oxygen produced can contain up to 10% ozone and pure ozone can be obtained by liquifaction of
the mixture followed by fractional distillation (O2, b.p. 90 K; O3, b.p. 161 K).
|


 .
. than H2SO4 electrolysis, since even the conditions can't be agreed (gives higher yields as well).
than H2SO4 electrolysis, since even the conditions can't be agreed (gives higher yields as well).