| Pages:
1
2 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
O3?
Ozone is an interesting oxidiser, but its synthesis is difficult and inefficient; pity it can't be formed by a contact process like the catalytic
oxidation of so2 on pt. gauze.
If this gas could be produced simply and cheaply it would be a boon to headcases like us. Our favourite acids(hno3-h2so4)could be easily made, with a
minimum of reagents and glassware.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
2KMnO4 + H2SO4 -> Mn2O7 + K2SO4 + H2O
Mn2O7 + xkJ -> 2MnO2 + O3
Dangerous reaction though, as the ozone evolution is usually explosive. On the other hand this is the only high-yield mass production possible.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
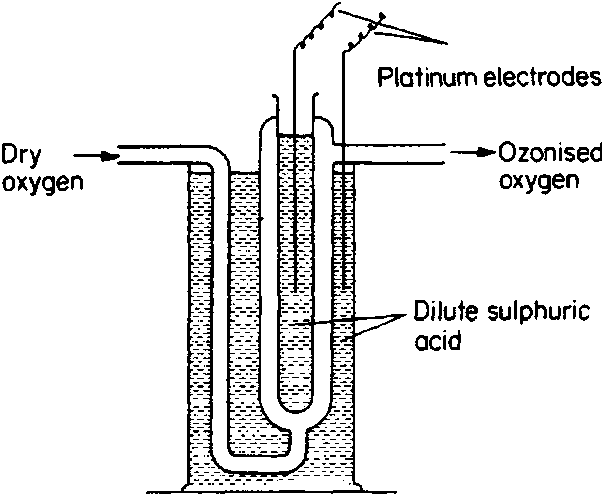
If you could live with low yields, you can also use one of the methods stated in C. Chamber's Modern Inorganic Chemistry. In case you didn't
download the book yet, here's an excerpt:
| Quote: |
Small quantities of ozone are produced when oxygen and air are subjected to an electrical discharge and it is, therefore, found in the neighbourhood
of working electrical machines. Probably a small quantity of atomic oxygen is initially produced; most of this recombines quickly to give oxygen,
O2, but a few atoms react to form ozone:
O2 + O --> O3
The ozone molecules also decompose by reaction with atomic oxygen, so that the actual concentration of ozone is small.
Ozone is formed in certain chemical reactions, including the action of fluorine on water [...] and the thermal decomposition of iodic(VII) (periodic)
acid. It is also formed when dilute (about 1 M) sulphuric acid is electrolysed at high current density; at low temperatures the oxygen evolved at the
anode can contain as much as 30% ozone.
Ozone is normally produced by the use of a silent electrical discharge and a number of ozonisers have been produced. Brodie's apparatus is shown in
outline in [file attachment].
Using a potential of approximately 20000 V the ozonised oxygen produced can contain up to 10% ozone and pure ozone can be obtained by liquifaction of
the mixture followed by fractional distillation (O2, b.p. 90 K; O3, b.p. 161 K).
|
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
O3
I think electrolysis of HClO4 yields 30% ozone together with O2 . .
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
A member with acces to HClO4 isn't going to need nor waste it to make O3.
Boiling a solution of a persulfate and electrolysis of dilute H2SO4 are other ways to get O3.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
vulture, could you provide some more data on the H2SO4 electrolysis for O3? Electrodes material, voltage, Amp/cmxcm...?
thanks
ORG
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
this is probaly of no help but i remeber there is some reaction involveing lithium that producd ozone
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
IIRC it's electrolysis of a warm 40% sulfuric acid solution on platina electrodes. The intermediate compound is peroxydisulfuric acid which
decomposes into ozone and yielding sulfuric acid again.
But I guess carbon electrodes would work too.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Ive posted this info before I'm sure somewhere.
Any dilute non oxidising acid/electrode system will produce ozone, for best results,
Dilute sulphuric acid of density 1.075 to 1.100,
Platinum-Iridium foil anodes.
Anode must be kept cold (ca -14C)
High current density bearing the above is the key, what this relys upon is the formation of oxygen radicals which then dimerise/trimerise. The higher
the current density the larger the proportion of ozone to oxygen in the exit gas.
Using a platinum foil 0.1mm thick by 11mm long embedded in glass then polished as the anode its possible to produce oxygen containing 17-28% of ozone.
Somewhere I had the current density this required, if I fall over it, I'll add this info. I think its in the 10's of amps per square cm
range.
Hot peroxide solutions are supposed to yeild some ozone when decomposed thermally or with catalysts, I'm not sure how well this would work in
comparason though.
[Edited on 10-7-2003 by Marvin]
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Well, electrolysis of HClO4 at high current density yields 30% O3, and I guess it's much more foolproof than H2SO4 electrolysis, since even the conditions can't be agreed (gives higher yields as well). than H2SO4 electrolysis, since even the conditions can't be agreed (gives higher yields as well).
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
If you have specific details, Id be interested to hear the full method Theoretic, but I suspect it amounts to the same method as Ive posted. It might
be slightly complicated by electrolytic reduction of the perchlorate at the other half of the cell though.
The different suggestions that use sulphuric acid dont amount to different conditions, so much as totally different methods. vulture's method
has a distinct advantages over ours if it produces a similar concentration of ozone. I have my doubts though.
I think silent electric is the way to go for anything but trivial amounts simply becuase cold electrolysis is so hard to get working large scale.
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
My(extremly old)chemistry book discribes(very breifly)the elctrical process.The yields are usally about 5 percent per pass in a half meter tube.But it
says it can be run over and over.
|
|
|
tryptamine
Harmless

Posts: 16
Registered: 20-3-2003
Member Is Offline
Mood: elated in love
|
|
easy O3
you can make a high yielding o3 generator with a neon sign transformer some wire, metal screen, and glass.
It works on the corona discharge and if sealed in a tube with a fan blowing over it produces a stream of O3 enriched air. One could use a bottle of O2
and run it through many generators or recirc it to get very high purity O3.
Actually one of the easier exotic reagents to make.
Look online for some home made O3 generators.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Interestingly, a problem with ozonisers, besides being very inefficient is that with moist airfeed they produce no2 as a contaminent. O3 oxidizes no2
to n2o5.
Moist air in an ozoniser affects conductivity, producing hot arcing; hot enough to dissociate n2. If moisture content could be controlled, nitric acid
might be condensed at the outlet.
It's a longshot, but stoichiometric quantities of air and water vapor might, in theory, in an ozoniser, produce nitric acid vapor alone. I love
the thought of air in-hno3 out. But will it work?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Corona isnt the best way for ozone, silent electric is a bit better, low density ionisation, less heat, less nitric oxides.
Daisy chaining ozonisers together wont get you more than about 30% using pure oxygen as input, as at this concentration its destroyed as fast as its
formed. Going beyond about 10% ozone output wastes a lot of energy.
For nitric acid, I did some calculations a while ago that done properly oxidation to nitric anhydride with ozone would add about 30% to the energy
requirement of making nitric acid by the arc process. This assumes an arc furnace in parrallel to an ozoniser fed with pure oxygen. It wouldnt be a
'simple' machine to build. A single chamber for both wouldnt work very well becuase ozone is thermally destroyed under the conditions
nitric oxide forms.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Marvin, it's nice to see someone put in some real mental effort. Ozone is of course unstable at all temps and would be lost oxidizing no-to-no2.
My ozonisers have been basic; neon
trans, windowglass, alu-foil and spacers.
An enclosed Jacob's Ladder fed by compressed air produced hno3 when its effluent dissolved in water. Both units fed by dry air and operated in
parallel would produce n2o5 when their outputs combine. Solid n2o5 could be collected if the apparatus was kept dry.
Both reactions are highly inefficient(uncatalysed)but the catalysed route for ozone is "out there" and waiting to be stumbled upon. It will
probably work like so2 oxidation on pt but without the thermally programmed desorption temp.
Cheap ozone might be used to oxidize nh3 and excess ozone might just produce n2o5.
Ok, it's idle speculation, without much thought; a redox reaction with o2 being reduced and oxidized; it does happen, though.
|
|
|
the_alchemist
Harmless

Posts: 4
Registered: 1-10-2003
Member Is Offline
Mood: spiffy
|
|
Ozone Generator
I actually consider myself somewhat of an authority on ozone generators, i've built one.
The idea is really simple. Electricity passes through a dielectric such as glass and breaks O2 into O1 to reform into O3.
What you'll need is a good long pyrex tube, or a long light bulb cut off at both ends is what I used, and a good long roll of thin copper wire,
some shorter lengths of really thick insulated copper wire, some electric tape, some O-rings, some alligator clips, a transformer (30 mA will do, go
to a sign shop these cost about 80 bucks if you shop around), and a ground or length of electrical cord if your house is the ground. If you are
looking for laboratory quantity/quality ozone then you'll have to feed the system with an oxygen tank, some folks go the extra mile and bubble
this through a drying agent along the way. You should have a local holox or something that retails tanks and regulators.
Basically you tightly wind the copper wire around the tube. The spacing of the wire will largely determine how much ozone is generated, but if you
wind it too close I imagine you'll get arching. Pass a peice of wire through the tube and attach it at both ends. Use electrical tape on both
ends, leaving a loop or bit of copper on the end of each wire for the alligator clips, which should be connected to the transformer on a thicker
insulated peice of copper wire and connected with O-rings.
Now i'll leave it up to you to figure out how to seal the ends. I used two PVC connectors on both ends to facilitate the seal and as caps to
which I connected the hoses, but any type of adhesive you try to use is just not going to last.
This produces enough ozone to melt rubber in short order and do serious damage to your health should you inhale it. The device I described does not
comply with EPA regulations and i'm not responsible for whatever you do to yourselves.
And by the way for use in synthetic reactions these machines must be set very low, as they can produce a hell of a lot of ozone, which as you no doubt
know can tear a molecule to shreds.
Happy oxidizing.
[Edited on 2-10-2003 by the_alchemist]
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
ozone
I think you could gererate some O3 from a cheap laser printer working in a small space.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, 10ppm or so....
In any case, below the approved MAC for ozone, which is very low.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
ozone
If you have got some ozone for a reaction then the reaction should be OK with oxygen and nitrogen. The ozone reacts and the oxygen and nitrogen are
blown away. The only problem is that it would take a month or more and all the kit would have to be up to speed.
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
This one is not very practical but here goes
| Quote: |
"Interaction of the solid acid (Bismuthic acid) with 40% hydrofluoric acid is violent, ozonised oxygen being evolved."
|
Mellor, 1939, Vol. 9, 657
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
related thread
http://www.sciencemadness.org/talk/viewthread.php?tid=8343
Reference papers on ozone producton here
http://www.sciencemadness.org/talk/viewthread.php?tid=1518&a...
.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | Ozone is an interesting oxidiser, but its synthesis is difficult and inefficient; pity it can't be formed by a contact process like the catalytic
oxidation of so2 on pt. gauze.
If this gas could be produced simply and cheaply it would be a boon to headcases like us. Our favourite acids(hno3-h2so4)could be easily made, with a
minimum of reagents and glassware. |
UV light works.
New or Improved Apparatus for the Production of Ozone by Means of
Phosphorus. C. R. Poulsen. Horsens, Denmark. English Patent 14,862, August
17, 1892.
The phosphorus is held in a glass cup fixed at the end of glass rod and placed in
a 10 per cent. solution of sulphuric acid, which is contained in a wide-mouth
bottle provided with a glass cap, through which the free end of the glass rod
projects. A perforated glass or porcelain plate is fixed below the
neck of the bottle though which the ozone escapes, whilst the phosphours acid formed
simultaneously is kept back. The bottle is filled to about one-half its volume with
the dilute acid, to which a little potassium permanganate is added to oxidize the
phosphorus acid. The apparatus is designed for inhaling of ozone in cases of
tuberculosis and of the diseases of the chest.
The Journal of the Society of Chemistry Industry.
Volume XII No. 3. March 31, 1893.
Back when the British pumped ozone into underground railroad
stations. A problem being passengers walked out smell of it.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | The apparatus is designed for inhaling of ozone in cases of
tuberculosis and of the diseases of the chest. |
As I understood it, the Pozone process produced a pretty contaminated ozone stream even with KMnO4 in the slurry . . .
Sick or well, I certainly wouldn't inhale it!
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: | | If you could live with low yields, you can also use one of the methods stated in C. Chamber's Modern Inorganic Chemistry. In case you didn't
download the book yet, here's an excerpt: |
Looking in my hard copy of Mellor's 16 volume opus V1 p886 this
looks like a slight variation of Brodie's ozonizer. (B. C. Brodie,
Phil. Trans., 162. 435, 1872.
Mellor notes that it is usually called Berthelot's "though the latter
designed it some years after Brodie."
Here be Brodie's ozonizer courtesy of Google.co/books —
http://tinyurl.com/33odbwt
|
|
|
| Pages:
1
2 |