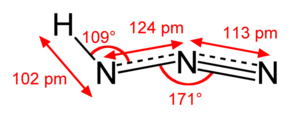
Hydrazoic acid
Hydrazoic acid, also known as hydrogen azide or azoimide, is a colorless, volatile, and explosive liquid at room temperature and pressure, with the chemical formula HN3.
Contents
Properties
Chemical
Hydrazoic acid is a weak acid, and will dissolve several metals, such as iron, magnesium, zinc. Antimony dissolves slowly in hydrogen azide.
Dissolution in the strongest acids produces explosive salts containing the H2N=N=N+ ion, for example:
- HN=N=N + HSbCl6 → [H2N=N=N+] + [SbCl6]−
Hydrazoic acid decomposes to nitrogen and hydrogen:
- 2HN3 → H2 + 3N2
Physical
Hydrazoic acid is a colorless liquid, with a strong and toxic smell. It is very soluble in water, as well as alcohol and ether. Its density is 1.09 g/cm3. Anhydrous hydrozoic acid melts at −80 °C and boils at 37 °C. It is a weak acid (pKa = 4.75).
Availability
Due to its long list of hazards, hydrazoic acid is not sold, and has to be prepared in the laboratory.
Preparation
Hydrazoic acid can be prepared by reacting a strong acid, such as sulfuric acid, with an azide, such as sodium azide or barium azide. The latter is preferred as the insoluble barium sulfate produced can simply be filtered out, giving a clean solution of hydrogen azide. It's recommended to produce diluted acid, as pure hydrazoic acid is prone to detonation.
Hydrazoic acid was originally prepared by the reaction of hydrazine with nitrous acid:
- N2H4+ HNO2 → HN3 + 2 H2O
As nitrous acid is already diluted, this reaction is safer. It can similarly be accomplished using nitric acid or concentrated hydrogen peroxide.
Projects
- Synthesis of 2-Furonitrile
- Silver, lead and mercury azide preparation
- Preparation of azides that hydrolyze in aqueous solutions, such as magnesium or antimony azide (should only be performed in aprotic solvents that do not react with hydrazoic acid, or with extremely small amounts of pure cold acid and metal or none at all)
Handling
Safety
Hydrazoic acid is volatile and highly toxic, similar in toxicity with cyanides, however it has no known antidote. It has a pungent smell and its vapors can cause strong headaches. In pure form it is prone to detonation, so it's best to work with diluted solutions. Azide salts carry the same dangers.
Storage
Hydrazoic acid should only be stored as diluted solutions, is closed bottles, in well ventilated areas or in a special storage cabinet. Should not be stored for long periods of time.
Disposal
Hydrazoic acid can be neutralized with an excess of sodium hydroxide. However, azide salts such as the sodium azide produced in this way are extremely toxic, and they should NEVER be disposed of as is, especially not down the drain. Sodium azide produced this way should either be destroyed with nitrous acid, crystallized out by evaporation and carefully heated to decomposition outside, or precipitated as a transition metal azide. Many transition metal azides are explosive, and these too must be very carefully disposed of.
References