Difference between revisions of "Sulfuric acid"
(→Properties) |
(→References) |
||
| Line 77: | Line 77: | ||
[[Category:Things that can kill you very quickly]] | [[Category:Things that can kill you very quickly]] | ||
[[Category:Hygroscopic compounds]] | [[Category:Hygroscopic compounds]] | ||
| + | [[Category:DEA List II chemicals]] | ||
Revision as of 11:01, 2 July 2016
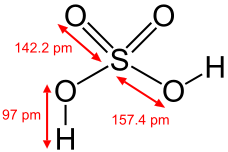
Sulfuric acid (alternative spelling sulphuric acid), represented by the molecular formula H2SO4, is one of the most important acids in chemistry and the most important chemical to industries in the world. It is the strongest easily available acid, with a pKa of -3.
Contents
[hide]Properties
Chemical properties
Sulfuric acid is a diprotic acid, it is able to give away two protons (H+). It first dissociates to form hydronium and hydrogen sulfate, with a pKa of -3, indicative of a strong acid:
- H2SO4 + H2O → H3O + HSO4−
The second dissociation forms sulfate and another hydronium ion from a hydrogen sulfate ion. It has a pKa of 1.99, indicative of a mid-strength acid, and occurs like this:
- HSO4− + H2O ⇌ H3O+ + SO42-
Concentrated sulfuric acid also has a strong oxidizing effect, converting nonmetals such as carbon and sulfur to carbon dioxide and sulfur dioxide, respectively, reducing sulfuric acid into sulfur dioxide and water in the process. This property is useful for producing large amounts of sulfur dioxide for use as a reducing agent if water is continually removed.
Sulfuric acid is sufficiently strong enough to protonate nitric acid, forming the nitronium ion, which can be used in a nitration mixture to make alkyl nitrates.
In organic chemistry, sulfuric acid is the most practical acid in most cases where a source of H3O+ ions are needed as it introduces the least amount of water. Organic compounds are often easily attacked by the nucleophiles left behind by the dissociation of acids such as HCl which leaves Cl- ions behind which can easily attack many organic compounds. However, the sulfate ions left behind by the dissociation of sulfuric acid are far less reactive than the ions left behind by most acids, it allows to protonate the reaction mixture without causing undesired side reactions in most cases.
When concentrated, it is strongly hygroscopic and has strong dehydrating properties. It can break down most organic molecules containing OH- groups to use them to form water, leaving only the carbon behind. This property is exploited in the famous "black snake" demonstration, where sulfuric acid dehydrates sucrose (table sugar), forming water with the hydrogen and oxygen atoms and leaving amorphous carbon behind.
Physical properties
Sulfuric acid is a oily liquid at room temperature. It is colorless, but often has a very light yellow color when slightly contaminated with iron ions. Even very small amounts of dissolved organic matter can change the color of concentrated sulfuric acid to pale yellow or pink, red, brown, and even black. It is commonly sold diluted at around 35% w/w with water as car battery acid and concentrated between 95% and 98% w/w as drain cleaner.
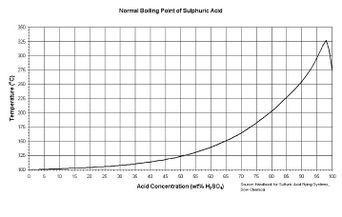
Sulfuric acid's boiling point raises with the concentration as described in this figure to the right. An azeotrope forms at 98% w/w.
At room temperature, sulfuric acid does not fume and has no smell. Hot sulfuric acid is known to fume profusely and smell like a mix of burnt matches and pure pain (this is because of its partial decomposition when hot; the smells correspond to sulfur dioxide and trioxide respectively).
Sources, production, and concentration
Sulfuric acid is a commonly used chemical for lead-acid batteries and drain cleaning. Battery acid can often be found at an auto store or a department store, and is approximately 35% sulfuric acid by weight. This is sufficient for most amateur chemists. If more concentrated sulfuric acid is desired, one can look in hardware stores for drain cleaner, which can be over 90% sulfuric acid by weight. For safety purposes, this concentration of sulfuric acid may have a dye in it. Other forms of sulfuric acid may be contaminated with various chemicals and will appear yellow, black, red.
For some amateurs, it can be hard to find concentrated sulfuric acid, with acid drain cleaners being banned (as a result of acid throwing or illicit drug manufacture) or very contaminated in some countries. So here we shall provide a concise list of available methods of making sulfuric acid.
The most well-tested method of concentrating sulfuric acid is described in a sub-article: Boiling the Bat.
- If you have technical grade sulfuric acid of concentrations from 80% to 94%, it can be converted to the pure compound by Zintl-Karyakin distillation. This process yields sulfuric acid of the highest quality and of concentration above the azeotrope. However, it is demanding in terms of glassware and very risky if performed at home. To perform this distillation, you need chromium trioxide or a dichromate salt (any will do, except ammonium: ammonium dichromate will decompose on heating, and you'll have green murky acid contaminated with chromium (III) oxide and chromium sulfate) that will work as an azeotrope breaker. Add this to a round-bottom flask, pour the acid in and connect it to an air-cooled condenser. Put thermal insulation (asbestos, rockwool) on the flask and start heating it. Discard the first few grams of the distillate, until its density reaches 1.84; collect every drop after that. This gives pure sulfuric acid with concentration above 98%. Beware of any spillage of hexavalent chromium, it's a carcinogen! If such a spillage occurs, neutralize it with any reducing solution such as potassium iodide.
It is possible to further concentrate sulfuric acid by adding sulfur trioxide, which reacts with the remaining water to form pure sulfuric acid. Sulfur trioxide can continue to be added to the solution to form oleum, which fumes in air to form sulfuric acid droplets. When an equimolar concentration of sulfuric acid and sulfur trioxide are added, it forms pyrosulfuric acid, which is a solid at room temperature.
Projects
- Producing metal sulfates
- Producing nitro compounds through nitration
- The dehydration of sucrose to produce elemental carbon
- Esterifications that require a dehydrating agent, such as that of methyl salicylate
- Making simple rayon fibers with Schweizer's reagent and cellulose
- Producing other concentrated acids by reaction of sulfuric acid with an anhydrous salt, such as in the production of fuming nitric acid and glacial acetic acid
Handling
Safety
While low concentration sulfuric acid is relatively safe to work with (under 40% w/w)), concentrated sulfuric acid (over 90% w/w) is extremely corrosive and dangerous. It does not only causes chemical burns, it also causes burns by dehydration of organic materials (like skin), destroying the molecules to form water with the -OH groups in them. Safety measures should be taken and all skin should be covered when working with concentrated sulfuric acid.When heating sulfuric acid, it is important to DO NOT OVERFILL THE FLASK. Concentrated sulfuric acid's volume increases by nearly 16% between 0 and 330°C, an overfilled flask will spill its content. Also, sulfuric acid, even diluted, tends to bump when it boils, accumulating heat to release a violent burst of steam from time to time. The use of boiling chips reduces this phenomena, but there is no way to stop it completely. It is advised to take measures to prevent spills, an anti-splash adapter with ground glass joint being a very convenient option.
Hot concentrated sulfuric acid may decompose to form sulfur dioxide and sulfur trioxide, which are toxic and corrosive, respectively. It fumes profusely when hot, the fumes consist of sulfuric acid droplets and a SOx mix. These fumes are very dangerous and a known lung carcinogen.
Storage
Sulfuric acid should be stored in closed bottles.
Disposal
Sulfuric acid can be neutralized with any base or carbonate, preferably calcium hydroxide or carbonate.