Difference between revisions of "Hexamine"
| (4 intermediate revisions by the same user not shown) | |||
| Line 66: | Line 66: | ||
| pKa = 4.89 | | pKa = 4.89 | ||
| pKb = | | pKb = | ||
| − | | Solubility = | + | | Solubility = 87.4 g/100 ml (20 °C)<br>86.7 g/100 ml (25 °C)<br>84.4 g/100 ml (60 °C)<ref>https://link.springer.com/chapter/10.1007/0-306-48639-3_17</ref> |
| SolubleOther = Reacts with strong acids<br>Soluble in [[chloroform]]<br>Slightly soluble in [[alcohol]]s, [[1,4-Dioxane|dioxane]], [[cyclohexanone]], [[glycerol]], [[toluene]]<br>Insoluble in [[petroleum ether]] | | SolubleOther = Reacts with strong acids<br>Soluble in [[chloroform]]<br>Slightly soluble in [[alcohol]]s, [[1,4-Dioxane|dioxane]], [[cyclohexanone]], [[glycerol]], [[toluene]]<br>Insoluble in [[petroleum ether]] | ||
| Solubility1 = 0.65 g/100 g (20 °C) | | Solubility1 = 0.65 g/100 g (20 °C) | ||
| Line 142: | Line 142: | ||
===Physical=== | ===Physical=== | ||
| − | Hexamine is very soluble in water and displays inverse solubility (is less soluble in hot water than cold). It is also soluble in [[chloroform]], [[ethanol]], [[methanol]], slightly soluble in [[acetone]], [[propanol]], [[isopropanol]], [[butanol]], [[isobutanol]], [[pentanol]], [[tert-amyl alcohol]] and poorly soluble in [[benzene]], [[benzyl alcohol]], [[carbon disulfide]], [[carbon tetrachloride]], [[cyclohexanone]], decahydronaphthalene, [[diethyl ether]], [[1,4-Dioxane|1,4-dioxane]], isoamyl alcohol, tetrachloroethane, trichloroethene, [[toluene]], [[xylene]] and practically insoluble in [[glycerol]], [[petroleum ether]]. The solid and solutions have a mild smell akin to fish, or cat food, which some people find this quite unpleasant. | + | Hexamine is very soluble in water and displays inverse solubility (is less soluble in hot water than cold). It is also soluble in [[chloroform]], [[ethanol]], [[methanol]], slightly soluble in [[acetone]], [[propanol]], [[isopropanol]], [[butanol]], [[isobutanol]], [[pentanol]], [[tert-Amyl alcohol|tert-amyl alcohol]] and poorly soluble in [[benzene]], [[benzyl alcohol]], [[carbon disulfide]], [[carbon tetrachloride]], [[cyclohexanone]], decahydronaphthalene, [[diethyl ether]], [[1,4-Dioxane|1,4-dioxane]], isoamyl alcohol, tetrachloroethane, trichloroethene, [[toluene]], [[xylene]] and practically insoluble in [[glycerol]], [[petroleum ether]]. The solid and solutions have a mild smell akin to fish, or cat food, which some people find this quite unpleasant. |
It is similar to the [[superbase]] [[DABCO]], mainly in structure and physical properties. | It is similar to the [[superbase]] [[DABCO]], mainly in structure and physical properties. | ||
| Line 148: | Line 148: | ||
==Availability== | ==Availability== | ||
Hexamine is widely used in camping fuel tablets, either pure or mixed with 1,3,5-trioxane. In both cases, a wax binding covers the small tablets, requiring the hexamine to be dissolved in water and the binders filtered off before use. | Hexamine is widely used in camping fuel tablets, either pure or mixed with 1,3,5-trioxane. In both cases, a wax binding covers the small tablets, requiring the hexamine to be dissolved in water and the binders filtered off before use. | ||
| + | |||
| + | Since around 2020, most chemical suppliers inside the EU stopped selling hexamine to the private individuals, or limit the amount they can sell, due to a new EU directive. Solid fuel tablets are still available without restrictions, though. | ||
==Preparation== | ==Preparation== | ||
| Line 158: | Line 160: | ||
*Preparation of primary alkyl or aryl amines from aryl or alkyl halides ([[Delépine reaction]]) | *Preparation of primary alkyl or aryl amines from aryl or alkyl halides ([[Delépine reaction]]) | ||
*Hexamine aromatic formylation (Duff reaction) | *Hexamine aromatic formylation (Duff reaction) | ||
| − | *RDX | + | *Stabilizer for photographic developers |
| − | *HMTD synthesis | + | *Synthesis of [[RDX]] and [[HMX]] |
| + | *[[Hexamethylene triperoxide diamine|HMTD]] synthesis | ||
*[[Lithium perchlorate]]-hexamine [[rocket fuel]] | *[[Lithium perchlorate]]-hexamine [[rocket fuel]] | ||
*Antiseptic | *Antiseptic | ||
| + | *Inhibitor against acids and hydrogen sulphide in metalworking | ||
| + | *Preservative for furs and skins | ||
| + | *Dyeing and artificial aging of wood | ||
==Handling== | ==Handling== | ||
| Line 168: | Line 174: | ||
===Storage=== | ===Storage=== | ||
| − | Hexamine should be | + | Hexamine doesn't require special storage conditions, though it should be kept in closed plastic bottles, at room temperature or lower, away from acids or corrosive vapors. |
===Disposal=== | ===Disposal=== | ||
| Line 190: | Line 196: | ||
[[Category:Easily prepared chemicals]] | [[Category:Easily prepared chemicals]] | ||
[[Category:Readily available chemicals]] | [[Category:Readily available chemicals]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 14:48, 18 November 2023
 Recrystallised hexamine and an example of it in tablet form.
| |
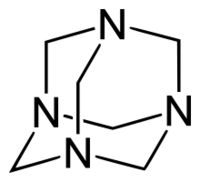 Hexamine structure
| |
| Names | |
|---|---|
| IUPAC name
1,3,5,7-Tetraazatricyclo[3.3.1.1]decane
| |
| Other names
1,3,5,7-Tetraazaadamantane
Aminoform Formin Hexamethylenetetramine Hexamine Methenamine Urotropine | |
| Properties | |
| C6H12N4 (CH2)6N4 | |
| Molar mass | 140.186 g/mol |
| Appearance | White solid |
| Odor | Weak fishy odor |
| Density | 1.33 g/cm3 (at 20 °C) |
| Melting point | 280 °C (536 °F; 553 K) (sublimes) |
| Boiling point | Sublimes |
| 87.4 g/100 ml (20 °C) 86.7 g/100 ml (25 °C) 84.4 g/100 ml (60 °C)[1] | |
| Solubility | Reacts with strong acids Soluble in chloroform Slightly soluble in alcohols, dioxane, cyclohexanone, glycerol, toluene Insoluble in petroleum ether |
| Solubility in acetone | 0.65 g/100 g (20 °C) |
| Solubility in benzene | 0.23 g/100 g (20 °C) |
| Solubility in carbon disulfide | 0.17 g/100 g (20 °C) |
| Solubility in chloroform | 13.4 g/100 g (20 °C) |
| Solubility in diethyl ether | 0.06 g/100 g (20 °C) |
| Vapor pressure | 6.1·10-4 mmHg at 25 °C |
| Acidity (pKa) | 4.89 |
| Thermochemistry | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 250 °C (482 °F; 523 K) |
| Related compounds | |
| Related compounds
|
Methylamine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexamine (or methenamine) is a solid, flammable organic compound that finds a use as a starting point for a few energetic materials. It has the chemical formula C6H12N4 or (CH2)6N4.
Contents
Properties
Chemical
Hexamine burns slowly, without producing smoke or melting. It is an effective reducing agent in energetic mixtures.
It is able to form salts, but at low pH the molecule breaks down into ammonia and formaldehyde.
Reactions with hydrogen peroxide form a very unstable organic peroxide explosive, similar to acetone peroxide, called HMTD.
Mild nitration forms hexamine dinitrate while complete nitration forms the powerful military explosive RDX. Nitration in the presence of acetic anhydride, paraformaldehyde and ammonium nitrate gives an even more powerful explosive, called octogen or cyclotetramethylene tetranitramine (HMX).
Hexamine can also be reacted with perchloric acid to form the moderately powerful explosive hexamine diperchlorate.
Tablets of hexamine set on fire can be used as heat sources in a home lab. It is included in some chemistry sets for this purpose. You can also buy them, and a simple apparatus for burning them, in a camping or survival store.
Physical
Hexamine is very soluble in water and displays inverse solubility (is less soluble in hot water than cold). It is also soluble in chloroform, ethanol, methanol, slightly soluble in acetone, propanol, isopropanol, butanol, isobutanol, pentanol, tert-amyl alcohol and poorly soluble in benzene, benzyl alcohol, carbon disulfide, carbon tetrachloride, cyclohexanone, decahydronaphthalene, diethyl ether, 1,4-dioxane, isoamyl alcohol, tetrachloroethane, trichloroethene, toluene, xylene and practically insoluble in glycerol, petroleum ether. The solid and solutions have a mild smell akin to fish, or cat food, which some people find this quite unpleasant.
It is similar to the superbase DABCO, mainly in structure and physical properties.
Availability
Hexamine is widely used in camping fuel tablets, either pure or mixed with 1,3,5-trioxane. In both cases, a wax binding covers the small tablets, requiring the hexamine to be dissolved in water and the binders filtered off before use.
Since around 2020, most chemical suppliers inside the EU stopped selling hexamine to the private individuals, or limit the amount they can sell, due to a new EU directive. Solid fuel tablets are still available without restrictions, though.
Preparation
Combinations of ammonia and formaldehyde create very pure hexamine, and the solution can be boiled to expel excess ammonia or fomaldehyde and to crystallize out the soluble hexamine. Hexamine can be further purified by sublimating it at high heat and depositing it on a cool surface. This can be accomplished with a bucket, a lid, and a heating source.
Projects
- Make formaldehyde
- Make methenamine silver stains
- Converted aryl halides to aryl aldehyde (Sommelet reaction)
- Preparation of primary alkyl or aryl amines from aryl or alkyl halides (Delépine reaction)
- Hexamine aromatic formylation (Duff reaction)
- Stabilizer for photographic developers
- Synthesis of RDX and HMX
- HMTD synthesis
- Lithium perchlorate-hexamine rocket fuel
- Antiseptic
- Inhibitor against acids and hydrogen sulphide in metalworking
- Preservative for furs and skins
- Dyeing and artificial aging of wood
Handling
Safety
Hexamine is not terribly toxic, so its major hazard comes from its high flammability. Also, because it does not melt or produce smoke while burning, it can be difficult to tell that samples are actually on fire, leading to accidental burns.
Storage
Hexamine doesn't require special storage conditions, though it should be kept in closed plastic bottles, at room temperature or lower, away from acids or corrosive vapors.
Disposal
Hexamine can be safely burned. Small amounts can be poured down the drain or in the ground.