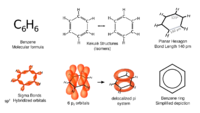Difference between revisions of "Benzene"
| Line 119: | Line 119: | ||
===Chemical=== | ===Chemical=== | ||
[[File:Benzene Representations.png|thumb|200px]] | [[File:Benzene Representations.png|thumb|200px]] | ||
| − | Benzene's aromatic ring is involved in complex reactions. At standard conditions, benzene is not very reactive, but it can react with halogens, alkenes, haloalkenes, organic acid anhydrides in the presence of a Lewis acid, (anhydrous [[aluminium chloride]], [[iron(III) chloride]]). | + | Benzene's aromatic ring is involved in complex reactions. At standard conditions, benzene is not very reactive, but it can react with halogens, alkenes, haloalkenes, organic acid anhydrides in the presence of a Lewis acid, (anhydrous [[aluminium chloride]], [[iron(III) chloride]]), process known as [[Friedel–Crafts reaction]]. |
The nitration of benzene occurs with a solution of concentrated [[sulfuric acid]] and [[nitric acid]]. | The nitration of benzene occurs with a solution of concentrated [[sulfuric acid]] and [[nitric acid]]. | ||
| + | |||
| + | Benzene is flammable and will burn in air, releasing copious amounts of soot. | ||
===Physical=== | ===Physical=== | ||
| Line 130: | Line 132: | ||
==Preparation== | ==Preparation== | ||
| − | Benzene can be prepared from the decarboxylation of [[sodium benzoate]] with [[sodium hydroxide]], by heating the mixture and condensing the resulting benzene. You can also use [[calcium oxide]] and [[benzoic acid]]. The decarboxylation method tends to produce [[biphenyl]] | + | Benzene can be readily prepared from the decarboxylation of [[sodium benzoate]] with [[sodium hydroxide]], by heating the mixture and condensing the resulting benzene. |
| + | |||
| + | :C<sub>6</sub>H<sub>5</sub>COONa + NaOH → C<sub>6</sub>H<sub>6</sub> + Na<sub>2</sub>CO<sub>3</sub> | ||
| + | |||
| + | You can also use [[calcium oxide]] and [[benzoic acid]]. The decarboxylation method tends to produce [[biphenyl]] and other side products, giving the condensate an orange or orange-reddish color. This can be removed with a simple distillation, giving clear benzene. | ||
Benzene can also be produced by the pyrolysis of plastic (usually polyethylene) and sodium hydroxide. This method produces benzene along with other hydrocarbons, so fractional distillation might be required.<ref>http://www.youtube.com/watch?v=JhUxaNesnxs</ref> | Benzene can also be produced by the pyrolysis of plastic (usually polyethylene) and sodium hydroxide. This method produces benzene along with other hydrocarbons, so fractional distillation might be required.<ref>http://www.youtube.com/watch?v=JhUxaNesnxs</ref> | ||
Revision as of 15:47, 20 May 2018
 Freshly distilled benzene
| |
 Structure of benzene
| |
| Names | |
|---|---|
| IUPAC name
Benzene
| |
| Preferred IUPAC name
Benzene | |
| Systematic IUPAC name
Benzene | |
| Other names
Benzol
Cyclohexatriene Phene Phenyl hydride Pyrobenzole | |
| Properties | |
| C6H6 | |
| Molar mass | 78.11 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet, aromatic |
| Density | 0.8765(20) g/cm3 |
| Melting point | 5.53 °C (41.95 °F; 278.68 K) |
| Boiling point | 80.1 °C (176.2 °F; 353.2 K) |
| 1.53 g/L (0 °C) 1.81 g/L (9 °C) 1.79 g/L (15 °C) 1.84 g/L (30 °C) 2.26 g/L (61 °C) 3.94 g/L (100 °C) 21.7 g/kg (200 °C, 6.5 MPa) 17.8 g/kg (200 °C, 40 MPa) | |
| Solubility | Miscible with glacial acetic acid, acetone, carbon disulfide, CCl4, chloroform, diethyl ether, toluene, xylene Immiscible with cyclohexane, heptane, hexane, pentane |
| Solubility in diethylene glycol | 52 g/100 g (20 °C) |
| Solubility in ethylene glycol | 5.83 g/100 g (20 °C) 6.61 g/100 g (40 °C) 7.61 g/100 g (60 °C) |
| Vapor pressure | 12.7 kPa (25 °C) 24.4 kPa (40 °C) 181 kPa (100 °C) |
| Thermochemistry | |
| Std molar
entropy (S |
173.26 J·mol-1·K-1 |
| Std enthalpy of
formation (ΔfH |
48.7 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | −11.63 °C (11.07 °F; 261.52 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
930 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Toluene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
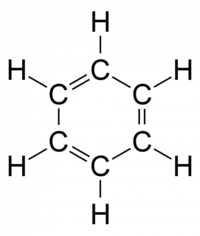
Benzene is an organic chemical compound with the molecular formula C6H6. It is the simplest aromatic hydrocarbon.
Contents
Properties
Chemical
Benzene's aromatic ring is involved in complex reactions. At standard conditions, benzene is not very reactive, but it can react with halogens, alkenes, haloalkenes, organic acid anhydrides in the presence of a Lewis acid, (anhydrous aluminium chloride, iron(III) chloride), process known as Friedel–Crafts reaction.
The nitration of benzene occurs with a solution of concentrated sulfuric acid and nitric acid.
Benzene is flammable and will burn in air, releasing copious amounts of soot.
Physical
Benzene is a colorless and highly flammable liquid with a sweet smell. It is not miscible in water, but it is in many other organic solvents, such as haloalkanes, ethers, ethanol. Benzene will dissolve certain plastic materials, such as polystyrene. It is also a good solvent for sulfur.
Availability
Benzene is available at organic chemical suppliers, but because of its environmental hazards it tends to be somewhat overpriced. In most countries it has been phased out from most products and it's also hard to find as a pure reagent.
Preparation
Benzene can be readily prepared from the decarboxylation of sodium benzoate with sodium hydroxide, by heating the mixture and condensing the resulting benzene.
- C6H5COONa + NaOH → C6H6 + Na2CO3
You can also use calcium oxide and benzoic acid. The decarboxylation method tends to produce biphenyl and other side products, giving the condensate an orange or orange-reddish color. This can be removed with a simple distillation, giving clear benzene.
Benzene can also be produced by the pyrolysis of plastic (usually polyethylene) and sodium hydroxide. This method produces benzene along with other hydrocarbons, so fractional distillation might be required.[1]
Best plastic material however is polyethylene terephtalate (PET), which is readily available. You will first need to hydrolyze it with sodium hydroxide in a high boiling point solvent, such as in ethylene glycol, which gives sodium terephtalate, which can be decarboxylated to benzene using sodium hydroxide. The resulting product is an orange-reddish liquid with lots of basic water, which can be separated by using a separatory funnel, followed by distillation. The yield from this reaction however, is lower than that from sodium benzoate (~15% compared to 45-60%), and plenty of terephtalate can get charred if the temperature is too high. Lots of side products are produced, which may clog the distillation apparatus.[2][3][4]
CHROMIUM has published a pdf on Sciencemadness library about many methods of synthesizing benzene, gathered from various members.[5]
Projects
- Napalm
- Toluene synthesis
- Chlorobenzene synthesis
- Nitrobenzene synthesis
- Sulfur extraction
Handling
Safety
Benzene is a strong carcinogen, so work must be performed in a fume hood or outside. Protection gear is required, as it can penetrate skin. If possible, use toluene or xylene, which are cheaper, safer and more widely available.
Storage
Benzene must be stored in closed bottles, away from any source of heat.
Disposal
Benzene can be destroyed using Fenton's reagent. If the oxidation is incomplete, phenol and biphenyl will result. If too much benzene is added, the resulting gasses will aerosolize some of the benzene, which is dangerous in an enclosed area, carrying both a fire/explosion hazard as well as inhaling dangerous and carcinogenic benzene fumes. This inconvenience can be reduced by adding the benzene dropwise or bubbling (non-flammable) gas-carried benzene through a gas diffusing stone through the Fenton solution to limit vapor escape. Either perform the neutralization in a fumehood, or outside, preferably the latter. Photochemical neutralization, with the aid of a UV lamp will improve the yield of the oxidation process.
Benzene can also be burned, but this will give off lots of soot, carbon monoxide, other aromatics and unburnt benzene vapors, unless it's done in a waste incinerator. If you really want to do this, do it outside, away from people.
References
- ↑ http://www.youtube.com/watch?v=JhUxaNesnxs
- ↑ https://www.youtube.com/watch?v=JuvczYeM3kk
- ↑ https://www.youtube.com/watch?v=uRTrjaAb4NA
- ↑ https://www.youtube.com/watch?v=Zh0RK0an8f4
- ↑ http://www.sciencemadness.org/member_publications/benzene_production.pdf