Difference between revisions of "Pentaerythritol tetranitrate"
| (3 intermediate revisions by the same user not shown) | |||
| Line 52: | Line 52: | ||
| BoilingPtC = 180 | | BoilingPtC = 180 | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| − | | BoilingPt_notes = ( | + | | BoilingPt_notes = (decomp. begins > 150 °C) |
| Density = 1.77 g/cm<sup>3</sup> (at 20 °C) | | Density = 1.77 g/cm<sup>3</sup> (at 20 °C) | ||
| Formula = C<sub>5</sub>H<sub>8</sub>N<sub>4</sub>O<sub>12</sub> | | Formula = C<sub>5</sub>H<sub>8</sub>N<sub>4</sub>O<sub>12</sub> | ||
| Line 62: | Line 62: | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| MeltingPt_notes = | | MeltingPt_notes = | ||
| + | | Odor = Odorless | ||
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| − | | Solubility = 0.0043 mg/100 | + | | Solubility = 0.0043 mg/100 ml (at 20 °C)<ref>Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4195</ref> |
| SolubleOther = Very soluble in [[acetone]]<br>Soluble in [[benzene]], [[toluene]]<br>Slightly soluble in [[methanol]] | | SolubleOther = Very soluble in [[acetone]]<br>Soluble in [[benzene]], [[toluene]]<br>Slightly soluble in [[methanol]] | ||
| Solubility1 = 25.4 g/100 g (20 °C) | | Solubility1 = 25.4 g/100 g (20 °C) | ||
| Line 70: | Line 71: | ||
| Solubility2 = 10.619 g/100 g | | Solubility2 = 10.619 g/100 g | ||
| Solvent2 = ethyl acetate | | Solvent2 = ethyl acetate | ||
| − | | VaporPressure = | + | | VaporPressure = ~0 mmHg |
}} | }} | ||
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| Line 95: | Line 96: | ||
| ExternalMSDS = [http://www.detotec.com/pdf/petn.pdf detotec] | | ExternalMSDS = [http://www.detotec.com/pdf/petn.pdf detotec] | ||
| FlashPt = | | FlashPt = | ||
| − | | LD50 = | + | | LD50 = 25,000 mg/kg (rat, oral) |
| LC50 = | | LC50 = | ||
| MainHazards = Explosive | | MainHazards = Explosive | ||
| Line 123: | Line 124: | ||
===Explosive=== | ===Explosive=== | ||
| − | Pentaerythritol tetranitrate is one of the most powerful explosive materials known, with a relative effectiveness (RE) factor of 1.66. | + | Pentaerythritol tetranitrate is one of the most powerful explosive materials known, with a relative effectiveness (RE) factor of 1.66. Its average detonation velocity is 8400 m/s. |
PETN is rarely used as a pure compound in explosive devices, instead it is mixed with various plasticizers, to form plastic explosives. When mixed with with [[RDX]], binders (styrene-butadiene e.g.), plasticizers (such as n-octyl phthalate or tributyl citrate), antioxidants (N-phenyl-2-naphthylamine) and dyes (Sudan I or IV) it forms a very powerful plastic explosive called Semtex, widely used by various countries of the former Soviet block. | PETN is rarely used as a pure compound in explosive devices, instead it is mixed with various plasticizers, to form plastic explosives. When mixed with with [[RDX]], binders (styrene-butadiene e.g.), plasticizers (such as n-octyl phthalate or tributyl citrate), antioxidants (N-phenyl-2-naphthylamine) and dyes (Sudan I or IV) it forms a very powerful plastic explosive called Semtex, widely used by various countries of the former Soviet block. | ||
| Line 130: | Line 131: | ||
==Availability== | ==Availability== | ||
| − | PETN is sold in pharmacies as a vasodilator, as treatment for heart conditions. The heart medicine Lentonitrat is nearly pure PETN. | + | PETN is sold in small amounts in pharmacies as a vasodilator, as treatment for heart conditions. The heart medicine Lentonitrat is nearly pure PETN. |
==Preparation== | ==Preparation== | ||
| − | PETN can be prepared by nitrating [[pentaerythritol]] using concentrated [[nitric acid]].<ref>http://www.google.com/patents/US2370437</ref> | + | PETN can be prepared by nitrating [[pentaerythritol]] using concentrated [[nitric acid]] under controlled conditions.<ref>http://www.google.com/patents/US2370437</ref> |
==Projects== | ==Projects== | ||
*Make blasting charges | *Make blasting charges | ||
| + | *Heart medication | ||
==Handling== | ==Handling== | ||
| Line 157: | Line 159: | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=22698 Question about PETN] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=22698 Question about PETN] | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=63225 PETN recrystallisation procedure for LOW density] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=63225 PETN recrystallisation procedure for LOW density] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=6903 PETN-based Cast Explosives] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=154137 initiate petn] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
Latest revision as of 17:47, 31 July 2023
| |
| Names | |
|---|---|
| IUPAC name
[3-Nitrooxy-2,2-bis(nitrooxymethyl)propyl] nitrate
| |
| Other names
Pentaerythrityl tetranitrate
Pentrite Pentrit | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C5H8N4O12 | |
| Molar mass | 316.14 g/mol |
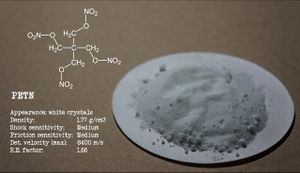
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.77 g/cm3 (at 20 °C) |
| Melting point | 141.3 °C (286.3 °F; 414.4 K) |
| Boiling point | 180 °C (356 °F; 453 K) (decomp. begins > 150 °C) |
| 0.0043 mg/100 ml (at 20 °C)[1] | |
| Solubility | Very soluble in acetone Soluble in benzene, toluene Slightly soluble in methanol |
| Solubility in acetone | 25.4 g/100 g (20 °C) |
| Solubility in ethyl acetate | 10.619 g/100 g |
| Vapor pressure | ~0 mmHg |
| Hazards | |
| Safety data sheet | detotec |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
25,000 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Erythritol tetranitrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentaerythritol tetranitrate or PETN is an explosive material, widely used in military applications, mainly due to its good stability and general performances. It is a nitrated organic compound, with the chemical formula C5H8N4O12.
Contents
Properties
Chemical
PETN will burn if ignited in air, releasing carbon dioxide, water vapor and soot.
PETN does not hydrolyze in water at room temperature, though this changes at high temperatures.
Physical
PETN is a white crystalline solid, insoluble in water, but soluble in acetone and dimethylformamide (40 g/100 g at 40 °C), as well as benzene and toluene. It also shows good solubility in ethyl acetate (10.619 g/100 g EtOAc).[2] It is also slightly soluble in alcohols, such as methanol and ethanol.
Explosive
Pentaerythritol tetranitrate is one of the most powerful explosive materials known, with a relative effectiveness (RE) factor of 1.66. Its average detonation velocity is 8400 m/s.
PETN is rarely used as a pure compound in explosive devices, instead it is mixed with various plasticizers, to form plastic explosives. When mixed with with RDX, binders (styrene-butadiene e.g.), plasticizers (such as n-octyl phthalate or tributyl citrate), antioxidants (N-phenyl-2-naphthylamine) and dyes (Sudan I or IV) it forms a very powerful plastic explosive called Semtex, widely used by various countries of the former Soviet block.
PETN is more difficult to detonate than primary explosives: dropping or igniting a block of PETN will not result in an explosion (at atmospheric pressure it is difficult to ignite and burns slow), however it is more sensitive to shock and friction than other secondary explosives such as TNT or tetryl.
Availability
PETN is sold in small amounts in pharmacies as a vasodilator, as treatment for heart conditions. The heart medicine Lentonitrat is nearly pure PETN.
Preparation
PETN can be prepared by nitrating pentaerythritol using concentrated nitric acid under controlled conditions.[3]
Projects
- Make blasting charges
- Heart medication
Handling
Safety
PETN is a vasoldilator and ingestion should be avoided.
Storage
PETN should be stored in closed containers. Urea is sometimes added as a stabilizer.
Disposal
PETN can be safely neutralized with Fenton's reagent. Diluted solutions of PETN should be added slowly, to prevent splashing.
Powdered iron can be used as a reducing agent.[4]
References
- ↑ Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4195
- ↑ http://pubs.acs.org/doi/abs/10.1021/j150566a032
- ↑ http://www.google.com/patents/US2370437
- ↑ http://pubs.acs.org/doi/abs/10.1021/es7029703?journalCode=esthag
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Nitrates
- Nitrated organic compounds
- Energetic materials
- High explosives
- Secondary explosives
- Things that can kill you very quickly