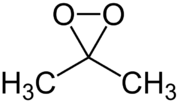
Dimethyldioxirane
| |
| Names | |
|---|---|
| IUPAC name
3,3-Dimethyldioxirane
| |
| Other names
Dimethyl dioxirane
DMDO Murray's reagent | |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.08 g/mol |
| Appearance | Unknown in pure form |
| Odor | Odorless |
| Melting point | Unknown |
| Boiling point | Unknown |
| Solubility | Soluble in acetone |
| Hazards | |
| Safety data sheet | None |
| Related compounds | |
| Related compounds
|
Acetone peroxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyldioxirane (DMDO), also referred to as Murray's reagent, is a dioxirane derived from acetone and can be considered as a monomer of acetone peroxide. It is a powerful yet selective oxidizing agent which finds use in organic synthesis.
Contents
[hide]Properties
Chemical
DMDO can be used to oxidize alkenes to epoxides.
DMDO displays good selectivity for olefins. Typically, electron deficient olefins are oxidized more slowly than electron rich ones.
DMDO will also oxidize several other functional groups. For example, DMDO will oxidize primary amines to nitro compounds and sulfides to sulfoxides.
In some cases, DMDO will even oxidize unactivated C-H bonds, such as those from tertiary carbons.
DMDO can also be used to convert nitro compounds to carbonyl compounds (Nef reaction).
Physical
Since the compound has not been isolated in pure form, its physical properties remain unknown so far.
Availability
DMDO is not commercially available because of its instability and has to be prepared in situ.
Preparation
DMDO can be prepared by the reaction of acetone with Oxone, where the potassium peroxymonosulfate is the active ingredient.[1]
The preparation of DMDO is rather inefficient (typical yields < 3%) and typically only yields a relatively dilute solution in acetone (only up to approximately 0.1 M).
Projects
- Make epoxides
- Make nitro compounds
Handling
Safety
DMDO is only stable in diluted solutions. It is a powerful oxidizer and will oxidize many organic compounds.
Storage
Solutions are stable under refrigeration (−10 to −20 °C) for up to a week. The rate of decomposition will increase upon exposure to light or heavy metals.
Disposal
Addition of a reducing agent will neutralize the compound.