| Pages:
1
2 |
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
How do you draw benzene rings?
| Quote: | So, benzene. When you first put pen to a blank piece of paper, you have a number of choices to make. Do you draw the hexagon so that its top and
bottom are parallel to the top of the piece of paper, or so that the left and right sides are parallel to the left-hand side of the piece of paper? Or
put more simply, is your hexagon sitting on a single point, or is it resting comfortably on one of its sides? Of course, if the benzene ring is part
of a larger structure, you might be forced to choose one orientation over another. But if you’re just drawing a single solitary benzene ring, or if
you’re not constrained in any way by the bigger picture, do you always start by drawing your benzene ring the same way up?
Orientation aside, once you’ve made that decision, which way do you move the pen? Do you always follow the same sequence of strokes? Do you lift the
pen from the paper before completing the hexagon and going on to the double bonds? After giving it some thought, I’m pretty sure I always draw my
benzene rings the same way — and if you’d asked me to describe how I did it without actually going through the motion, I’m not sure I would have
got the answer right. I guess it’s because it’s second nature; I’ve done it so many times it just happens, and the way I do it is tucked away in
my subconscious somewhere. So, for me, I always sit my benzene rings on a point (if I have a choice) and I lift my pen off the paper twice before even
completing the hexagon. And I’m pretty sure I always draw the double bonds in the same order and direction as well. Full gory details shown in the
picture. |
(Source: https://stuartcantrill.com/2011/09/12/drawing-conclusions/)
[Edited on 181022 by fusso]
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
I draw a line that is goes from the top of a line to the next. Then I draw a 60 degree angle and line of similar length. Rinse and repeat three times.
Is there a better way?
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If you imagine a clock face... I join 12 to 2, 2 to 4, 4 to 6 and so on.
Then I draw a (rough) circle in the middle of it
Except sometimes...
In molecules with linked rings, it's sometimes easier to draw some of the hexagons "sideways" (on a clock you would start by drawing 11 to 1 then 1 to
3 etc).
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I am lazy. A circle inside a hexagon. Done. https://www.quora.com/Why-is-there-a-circle-drawn-in-the-mid...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That's not even lazy- it's more accurate than writing alternating single and double bonds. Unless you're trying to draw a reaction mechanism, at
least...
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Left, Right, Hat, Pants, circle or bonds as needed.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Its more correct to draw a circle inside if there is a resonance, for example in benzene, but individual double bonds must be drawn if there is no
resonance, like in C60.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I draw separate bonds but make a point of changing their position in subsequent stages of the reaction until some intermediate locks them in place.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
I draw it so that it is sitting on a flat side rather than on a single CH group.
I usually draw a circle inside the hexagon rather than draw the double-bonds
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by VSEPR_VOID  | | Its more correct to draw a circle inside if there is a resonance, for example in benzene, but individual double bonds must be drawn if there is no
resonance, like in C60. |
If C60 had no "long range" resonances it would be colourless like benzene.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I draw mine more or less the same as j_sum1, its a flawless method.
First you draw 2 parallel lines vertically, this sets the size of your ring, then you simply put 2 dots at the top and bottom where you want the 2
other corners to be and then fill in where the lines need to be followed by drawing the double bonds (i prefer double bonds to the circle cause it
looks prettier).
A perfect benzene ring everytime.
[Edited on 28-10-2018 by Assured Fish]
Sufficiently advanced science is indistinguishable from madness.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
They're actually much easier to draw if you just draw them on edge.
Then they're just a straight line 2 bonds long.
It's all a matter of perspective.
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
I've got one tattoo'd on the back of my neck... didn't do it myself... that would be super stupid
Hyperbole be thy name
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
So drawing benzene like this is cursed according to reddit...
https://www.reddit.com/r/chemistrymemes/comments/k5h75w/the_...
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
User name says it all.
Kekule will come back from the dead and hunt them down. The benzene ring is made from one snake not three!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Haha, nice one.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I have recently discovered, that when I don't take my hand off the sheet, I draw straight rings. Altough, when I draw it line by line, it comes out
uneven as shit 
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I realized that I draw benzene rings very strangely. I draw mine on point in three separate strokes. Here's a diagram to demonstrate:
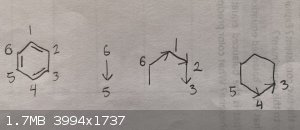
First, I draw the left side 6→5. Next, I pick up my pencil and draw 6→1→2→3. Then I move my pencil again and finish the last two bonds,
5→4→3. It's probably because I'm left handed. It just feels comfortable for me to "push" my pencil from left to right on the page. I always draw
the double bonds the way I show in the picture, starting with 6→5, then 1→2 and then 4→3, unless the other way is required for a mechanism.
Also... this thread reminds me of a meme I made a couple years ago  : :
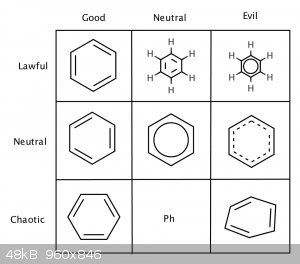
The whole thing was made in ChemDraw
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I prefer the Greek letter phi to the Ph abbreviation, personally. But it depends on why I'm drawing it- if it's part of a larger structure, then phi.
If I am drawing a mechanism, alternating single/double bonds with explicit hydrogens.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DraconicAcid  | | I prefer the Greek letter phi to the Ph abbreviation, personally. But it depends on why I'm drawing it- if it's part of a larger structure, then phi.
If I am drawing a mechanism, alternating single/double bonds with explicit hydrogens. |
Huh, I've never seen
anyone abbreviate phenyl as phi. Also, upon reconsidering my meme, I suppose I should have technically written PhH instead of Ph, since it is benzene,
not phenyl.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@tex the usual format for such alignment charts is having lawful~chaotic as x axis. Why are LG & NG there?
[Edited on 201210 by fusso]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Texium (zts16)  | Quote: Originally posted by DraconicAcid  | | I prefer the Greek letter phi to the Ph abbreviation, personally. But it depends on why I'm drawing it- if it's part of a larger structure, then phi.
If I am drawing a mechanism, alternating single/double bonds with explicit hydrogens. |
Huh, I've never seen
anyone abbreviate phenyl as phi. Also, upon reconsidering my meme, I suppose I should have technically written PhH instead of Ph, since it is benzene,
not phenyl. |
It's a habit I picked up from a collection of old chemistry books. When I was in grad studies, it confused the heck out of my supervisor, who had
never seen it used.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Quote: Originally posted by DraconicAcid  | | I prefer the Greek letter phi to the Ph abbreviation, personally. But it depends on why I'm drawing it- if it's part of a larger structure, then phi.
If I am drawing a mechanism, alternating single/double bonds with explicit hydrogens. |
do you use the capital phi or the lowercase?
I've never seen that syntax before. That's really interesting
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah I’m well aware of
that and I’m not sure why I did it reversed when I made it a few years ago
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I do it as below. I don't know if it's a common way or not, but it's the fastest way to produce a readable result for me. Just for fun I timed myself
drawing 10 of them, and it takes me 1.5 second per benzene!
But the real test is drawing a 7-, 8- or 9-membered rings. Those are a bitch to get right...
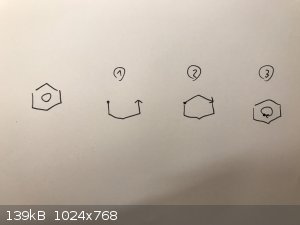
|
|
|
| Pages:
1
2 |