| Pages:
1
2 |
mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
4-methoxyphenol syn using hydroquinone methyl iodide
Hi
Can methyl iodide be used to methylate hydroquinone, in order to obtain 4-methoxyphenol, or would the iodide ion cause side reactions. If possible
does anyone have any experimental details on using methyl iodide. I suppose lithium carbonate could be used to remove the acidic proton, but could
someone tell me the solvent to use, reaction conditions, quantities and time etc.
Thanks
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I think you would get a lot of dimethylated product.
Hydroquinone can be selectively monomethylated by strong acid in R-OH where R is the alkyl group you wish to substitute onto one hydroxyl group in the
presence of some benzoquinone. See British patent 1,557,237. There are other patents out there that make subtle modifications to this procedure, but
this should get you started. I admit though I have no experience with this reaction.
[Edited on 25-1-2008 by smuv]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
interesting article
check this out:
Attachment: MeOphenol green.pdf (164kB)
This file has been downloaded 4411 times
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
There absolutly no need of using such a reageant for monomethylation of hydroquinone!
The patent mentionned above works wonders. Just add conc. H2SO4 to MeOH with stirring, add your hydroquione, then cat. amount of benzoquinone
(easily made in 1H from hydroquione, H2O2 and cat. I2), and either reflux for 4h or stir at room temp for 24H. Than evaporate half the MeOH, add
equivalent amount of brine, extract the black oil with DCM, wash with water and brine, dry evaporate the solvant, and vacuum distill the remaining
dark oil, using a condenser without cooling water. The p-MeO-phenol passes as a clear yellow oil between 140 and 150°c at water pump pressure. Be
carefull, keep a hot air gun or hair dryer at hand to keep the setup warmish to avoid cristn of the oil. There will be a largish amount of residu
left, but yields are always in the 80's after distn. Then recrysatllized the material from ethylacetate/pet ether to obtain beautifull cristals of
very pur material. Have a look: 
Distilled oil:

After cristalization:

After recrystallization:


Keep your MeI for better uses.
The patent suggets neutralizing the reaction mixture, and then directly distilling everything. But it can tricky to get the exact amount of base in
there, and so material is trapped in the formed Na2SO4, even after washing with fresh MeOH. ALso, no need of doing a A/B with the phenol, it incredbly
messy, you get a very dark emuslion with is impossible to break, and yields don't change after distn. Vac. distiling this compound is much more easy
than what has been suggetsed previously. I can't understand why people spent so much time/effort to directly recrystalize the crude oil, it would
take 1/10th the effort to build a vac. source and open doors to beautifull cristals.
[Edited on 26-1-2008 by Klute]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Very impressive results .....what is the yield?.....there is also another page to read is not already...............solo
http://erowid.org/archive/rhodium/chemistry/4-methoxyphenol....
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by solo
Very impressive results .....what is the yield? |
Never under 70%, most of the time around 80% (did the reaction 3 times..) after distn and recrystalization. So the reaction itself must not be far
from quantitative!
A short reflux (4H seems as effective as a 24H RT stirring. But stirring for 12h after reflux seems to lower the yields. Also, adding the BQ
gradually *could* give slighter better yields, in both the reflux and RT procedure. Didn't do any comparative work.
I usually used 10mL conc. H2SO4 with 100mL MeOH for 10g HQ and 1g BQ, but I think the amount of MeOH can be diminished, aswell as the amount of BQ.
Everything is dirt cheap anyway.
[Edited on 26-1-2008 by Klute]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
US 4469897 is also interesting.
Essentially, methanol CuCl2 and hydroquinone were refluxed for 8 hours while air was allowed to percolate through solution using a bubbler; 75%
conversion 90% selectivity (4-methoxyphenol).
Anyone care to rationalize how this works? I could see how the Cu++ could oxidize hydroquinone to 1,4-benzoquinone and then be regenerated by the
flowing air. From there though...I am stumped.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Maybe the CuCL2 could act as a weak Lewis acid, as per the paper chemrox posted, promoting the same mechanism.
Thanks BTW, I was curious as to what the mechanism was with HQ/BQ, there was this issue a while back as too wich species was alkylated, the
benzoquinone or the hydroquione.
BTW, I plan on trying out this reaction one other hydroquinone, when I can make/buy some. Not sure what substarte to use though yet.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Are you asking this because you would like to use a substituted hydroquinone and wonder if you need to use a corresponding benzoquinone?
The patent shows that the benzoquinone and hydroquinone if ring substituted should have the same R group. This implies that the benzoquinone gets
alkylated and in the process oxidizes hydroquinone to the corrisponding benzoquinone.
|
|
|
mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
Thanks guys you have sent me in a totally different direction which looks really easy. Klute although I have read the patents, can you elaborate on
the quantities of reagents you used and why do you only evaporate half the methanol before adding brine.( As a saturated solution I take it)? Do you
try and separate unreacted hydroquinone prior to short bulb distillation under reduced pressure, or do you use the difference in boiling points to aid
purification or maybe purify product with toluene separation.
Hydroquinone bp 287-C
4-methoxyphenol bp 243-C at std pressure
PS nice pictures Klute
|
|
|
mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
Klute, just read you extract the black oil with DCM which I think would leave behind any unreacted HQ, am I right in thinking HQ is not soluble in
DCM.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The previous thread on the same issue:
https://sciencemadness.org/talk/viewthread.php?tid=6284
PS: It is nearly impossible to get useful yields of p-methoxyphenol from hydroquinone by (mono)methylation since p-methoxyphenol is more acidic than
hydroquinone. It would only be a total waste of MeI.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I evaporate half the MeOH before adding brine to avoid heating the phenols in a conc. solution of H2SO4, causing much more side reactions. This is
mentionned in the patent; they add an equal amount of water when half the MeOH is distilled, dist the binary mixture, add water etc I also did that at
oone point but you can't recyle the MeOH without another fractionnation. The seperation was easier, as when cooling the oil crashes out even before
adding brine.
Most of the HQ stays in the aq. and the washes, and some in the rediu I take it. Aslo, all the quinones and other shit stays in the flask, there is
quite a large residu after distn, but the p-MeO-Phenol seperates well you get shrap fractions. There's always a little amount of colored impurities,
even if I purged the setup with Ar before distn. Hence the reXlization.
I've already given the amounts I used, a few post back, gathered rather arbitraly from the patent. I'm pretty sure the amount of MeOH can be cut to a
reasonable extent, aswell as the amount of BQ. The number of runs they did to compare the ratios of reagents is very informative, pretty rare to see
such work in patents, my results seems to correspond to their, so I doubt it's BS.
@Smuv, yes this is what I had on mind. I wasn't sure if it was the carbocationic alkylated benzoquinone was actualy a alkylating species, or if it
was reduced to the phenol, or both. I think it's pretty unclear from the article:
"The next step is the reaction between DS [carbocationic alkylated benzoquinone] and BS [protonated hydroquinone] to
produce the product ES [protonated p-MeO-phenol] , and the cocatalyst is released as CS [carbocationic unalkylated benzoquione]."
This lead me to think the benzoquione is always regenerated as such, and isn't reduced to the phenol. But when using a substitued hydroquinone, could
it be possible that the unsub benzoquinone could oxidize the sub hydroquione to give respectively unsub hydroquinone and sub. benzoquinone? It would
depend of the oxidation potentials I guess, wich would depend on the substituants. Could anyone be able to clear this issue up? Nicodem?
In any case, I think if this reaction was to be tried with sub. HQ, I'd better just follow the patent and use the corresponding BQ; it's just for
the sake of curiosity regarding the mecanism.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Its just a matter of redox potentials and equilibrations among the hydroquinone, p-benzoquinone and methyl hemiacetals of p-benzoquinone. No
O-methylation or any type of alkylation is ever involved. p-Benzoquinone is in equilibrium with its hemiacetal which gets reduced to p-methoxyphenol
by hydroquinone which itself gets oxidazed to p-benzoquinone... and so on the cycle cycles until no more hydroquinone is left. At least that would be
the classical mechanistic interpretation of this reaction.
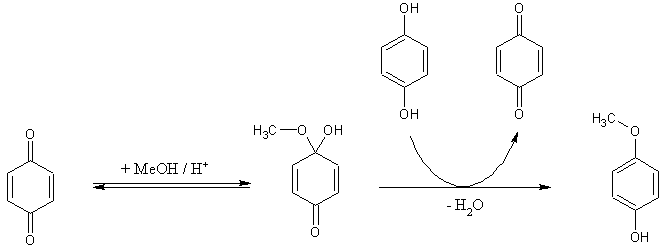
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
@Nicodem: In the article chemrox posted the authors pretty extensively explained the mechanism of a seemingly identical reaction (using a different
acid) however they made not mention of hemiacetal formation. I agree that what you have proposed does seem pretty textbook however why have the
authors of the above paper proposed such a novel mechanism? I guess what I am asking is, does the mechanism proposed in chemrox's paper seem valid
too (other than repeated erroneous drawings benzoquinone)?
I suppose though, in terms of how it applies to substituted hydroquinones, both proposed mechanisms would require an analogously substituted
benzoquinone.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information

what is it?
4-METHOXYPHENOL
SYNONYMS p-Hydroxyanisole; p-Methoxyphenol;
Hydroquinone monomethyl ether; Mono Methyl Ether Hydroquinone; Mequinol;
APPLICATIONS
It is used as an inhibitor in vinyl and acrylic monomers, especially for clear products and as an antioxidant. It is used as a stabilizer to inhibit
peroxide formation in ethers, chlorinated hydrocarbons and ethyl cellulose. It is also used as an intermediate to manufacture other stabilizers, dyes,
pharmaceuticals and plasticizers.
..........................source,
http://chemicalland21.com/specialtychem/perchem/4-METHOXYPHE...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by solo
It is used as a stabilizer to inhibit peroxide formation in ethers, chlorinated hydrocarbons and ethyl cellulose. |
That could be pretty interesting, for recylced ethers, redistilling them before use if need be. I wonder what the thereshold effective amount would
be. I guess I could just aswell use some hydroquinone though.
@Nicodem: thanks for that clarification. I think that was the mecanism suggested back then.
@smuv:I think in the article the mecanism is presented differently to explicit the role of the solid acid catalyst (they speak of "chemiabsorbed"
species). But I do find their mecanistic cycle a bit unclear....
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Clearly the authors were not specialized in organic chemistry or they would not make so many mistakes (indeed it is terrible that they continuously
write the structure of p-benzoquinone wrongly, but more so is terrible that the reviewers and editors never corrected them). The presence of the
destabilized carbocationic intermediate formed from p-benzoquinone protonation, as they draw it, is not just formally wrong (what they surely had in
mind is the O-protonated p-benzoquinone of which one of the resonance structures is indeed what they wrote) but in MeOH as solvent the hemiacetal
immediately forms by methanol addition. All would be well if they had drawn the O-protonated p-benzoquinone as the intermediate to such hemiacetal
formation (this mechanism of hemiacetal formation is described in most textbooks), but they insinuate that this carbocationic intermediate condenses
with protonated methanol (MeO<sup>+</sup>H<sub>2</sub> which is absurd. Why would an electrophilic center react with an electrophilic center?
which is absurd. Why would an electrophilic center react with an electrophilic center?
Anyway, it is just a matter of them not following the formal rules for writing down mechanisms and not having much insight in mechanistics. But what
they lack in insight they compensate with hard work. After all every mechanism is a just a hypothesis. Thus, they have all the right to propose their
own mechanism as long as it does not go against the theory (and here is where the reviewers failed to do a proper job). We all do mistakes, but it is
up to our peers to warn us about them...
PS: Using p-benzoquinone on a substituted hydroquinone should result in a mixture of the substituted p-methoxyphenol as major product and
p-methoxyphenol as minor product (besides the usual black tar and other crap). That is assuming the substituent is an alkyl. If the substituent is an
electron withdrawing group the reaction would be slower or fully inhibited (depending on the redox potential difference among the
substituted/unsubstituted p-benzoquinones).
[Edited on 28/1/2008 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Klute, I have considered this. However, overall the paper chemrox posted seems to be the same reaction as that of the British patent with a different
acid catalyst. I find it weird that 2 seemingly similar procedures are explained by such different mechanisms.
P.S. I am not trying to prove the patent writers or Nicodem wrong, I am just trying to figure things out.
Edit: I was writing my message as you posted Nicodem; thank you for expressing your view upon this subject. I guess it makes sense as when I was
drawing out the reaction mechanism for this (curved arrows and all) by the author's mechanism weird things were forced to happen.
[Edited on 28-1-2008 by smuv]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
IMPORTANT NOTE,
During a preparation of p-methoxyphenol via a similar procedure to Klutes, I encountered towards the end of the distillation of ethyl acetate I
noticed a strong odour of acetic acid and a dark red liquid that did not crystalise on cooling (however crystals deposited), I believe that when
heated in a the flask, p-methoxyphenol maybe strong enough to catalyse the hydrolysis of the ethyl acetate to acetic acid.
I strongly recommend removing the ethyl aceate solvent form the crude p-methoxyphenol on a hot water bath or rotary evaporator, do not use a mantle as
I did. Sloppy mistake.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I found this when researching uses for 4-methoxyphenol http://en.wikipedia.org/wiki/Mequinol
Apparently in combination with "Tretinoin" it can cure vitiligo and liver spots.
I suppose it is also a good starting point for some substituted phenethylamines...
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Indeed it is a melanocytoxic agent, killing the pigment-producing cells in the skin.
Crazyboy you only need to quickly brush the surface of this forum to find this compound (and similar) being used in skeletons of
phenethylamines/amphetamines. It's a shame really because it is a rewarding compound to make just for the nice crystals you can isolate. Also it
(together with the o-formyl derivative) can be used as building blocks for substituted heterocycles amongs other uses.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Well I just got finished performing this reaction using the ratios Klute posted, but I doubled everything. The reaction went very smoothly however it
used a considerable amount of methanol, DCM and hydroquinone but gave very little 4-methoxyphenol. I plant to repeat this reaction on a larger scale
but I will try to recycle some of the methanol/DCM. I will also add the hydroquinone more slowly. The only problem I ran into was when I was vacuum
distilling I tripped the surge protector on my power strip a few times and when I restarted the vacuum pump the mixture bumped considerably splashing
some of the impure compound into the collection flask. I don't have ethyl acetate or petroleum ether at the moment and recrystallization from
acetone/DCM is a nightmare. I think I will evaporate off all the solvent and synthesize some ethyl acetate.
And now pictures!
The reaction apparatus:

Reaction refluxing:

Extraction:

Crude crystals:

Short path vacuum distillation:

[Edited on 10-1-2010 by crazyboy]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for the pics!
Ouch I'm scared for that little pump there, no protection? It's pretty sure you'll have some product passing into the oil of the pump, especially
without a condenser..
At what temp does you phenol pass?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Quote: Originally posted by Klute  | Thanks for the pics!
Ouch I'm scared for that little pump there, no protection? It's pretty sure you'll have some product passing into the oil of the pump, especially
without a condenser..
At what temp does you phenol pass? |
Yeah, I won't be doing that again until I get a buchner flask or something to bubble the vapor through. Luckily though the vapor that got pulled into
the tube condensed in the first few inches and didn't make it to the pump, I'm more worried about the heat/moisture.
The product seemed to pass over at 140-150C but I had the paint stripper on the glass right where the thermometer bulb was so the temperature went to
160-165C.
|
|
|
| Pages:
1
2 |