| Pages:
1
2 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Ceric ammonium nitrate- uses and synthesis
The Ce(IV) ion is a powerful oxidiser in aqueous solution, stronger than chlorine, and capable of very interesting reactions, especially in organic
chemistry.
When its oxidising properties are to be used in preparations, it is most commonly employed as the salt ceric ammonium nitrate, or short CAN:
(NH4)2Ce(NO3)6
I think that this reagent has received much less attention among amateur chemists than it deserves.
The Organikum (22nd edition) has a preparation of p-tolualdehyde (p-methylbenzaldehyde) from p-xylene by oxidation of one of the methyl groups to the
aldehyde group with CAN.
A yield of 68% is claimed, which is very good for such an aldehyde preparation that easily can result in overoxidation to the acid when other
oxidisers are used.
This preparation is very simple and straightforward: 0,4 mol CAN in 200ml 50% aqueous acetic acid is heated with 0,1 mol p-xylene on the boiling water
bath under strong stirring and reflux for 20 min (soln becomes light yellow). After cooling down it is extracted thrice with ether, the pooled
extracts washed with Na2CO3 soln to remove acidic byproducts, dried with MgSO4 and distilled to separate solvent, product, and impurities.
I think this could work just as well for the oxidation of toluene to benzaldehyde. Isnt that something a lot of people here always want to do?
It also oxidises benzylic alcohols to the aldehyde, and phenols to quinones.
The wikipedia page on CAN is very informative:
http://en.wikipedia.org/wiki/Ammonium_cerium(IV)_nitrate
This useful chemical is, however, quite expensive (500g 109€ from Aldrich, cheapest grade) from most chemical suppliers, far more so than the other
well-known oxidants like permanganate and dichromate.
A method for its preparation at home would be something worth investigating.
A cheap source for cerium compounds exists: ceric oxide, CeO2, is used for polishing gems and glass and is cheap in this form.
Be sure to get the white quality, this is essentially pure CeO2.
I will buy 200g of such CeO2 soon.
As its use for polishing suggests, this oxide is strongly calcined (so that it consists of hard, abrasive crytals) and thus unreactive, just like
calcined alpha-Al2O3.
It does not dissolve in acids unless a reducing agent is present, according to Brauer, and then it goes in solution as the Ce(III) ion.
Wikipedia says that a solution of the Ce(NO3)6(2-) ion can be obtained by dissolving Ce2O3 in conc HNO3 (oxidation of the Ce(III) takes place)- this
has to be boiled according to Brauer.
After evaporation of most of the HNO3, ammonium nitrate solution can be added to form the salt, and this crystallized after repeated evaporation with
HNO3 (dont ask why, Brauer says it).
Brauer gives a rather tedious method for CeO2 dissolution using hydroquinone which gives problems because the benzoquinone byproduct must be gotten
rid of before further processing. A different and less problematic reducing agent seems to be necessary- I'd like to try formic acid.
Brauer further gives a procedure for crystallizing the CAN and an ether extraction of ceric nitrate from CAN in 6N HNO3 for complete separation from
other rare earth elements- not necessary for us if the starting material is pure.
We can stop after the crystallization of CAN from the HNO3 solution after addition of AN and repeated evaporation with HNO3.
Obviously the yield will be far from quantitative due to CANs high solubility (mother liquor can be further boiled down once to yield a second crop),
but the Ce values can always be recycled from the mother liquors (and spent CAN during further use in synthesis) simply by diluting with water and
adding oxalic acid while hot- the oxalates of the trivalent rare earths are all sparingly soluble, and the oxalic acid acts as the reducing agent as
well.
If someone wants to do this, read the chapter "Pure cerium compounds" in Brauer, it essentially contains a preparation of CAN but not an optimal one.
When I receive my CeO2 I will try this out and report here.
Has anyone here done preparative work with Ce(IV) compounds or knows about them? Woelen?
Any comments or tips towards synthesis of CAN?
[Edited on 20-11-2007 by garage chemist]
|
|
|
Xenoid
National Hazard
   
Posts: 776
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Oh! No! Please don't use CAN as an abbreviation for this chemical!
CAN is the pretty much standard abbreviation for Calcium-Ammonium Nitrate, a fertilizer which is a blend of about 20% CaCO3 and 80% Ammonium Nitrate.
Wouldn't CeAN or CEAN be more appropriate!
Regards, Xenoid
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Ethanol or H2O2 have been used as reducing agents when dissolving CeO2 in HCl or H2SO4, and also to reduce slightly acid solutions of Ce(IV).
Frederick Smith's Cerate Oxidimetry starts with a low grade of CeO2, 45% or 60% ceric oxide. These grades dissolve in HNO3, the 45% with
evolution of heat. I suspect that these grades are no longer obtainable, or if so only in box car quantities.
I'll dig the pamphlet out and type in the CAN production from it.
CAN is nearly insoluble in concentrated HNO3, while the REE(III) nitrates are somewhat soluble. Thorium, as the nitrate, would be the main
contaminate, these days thorium doesn't really show up in REE products.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have not really done preparative work with cerium salts, but I have some experience with the cerium(III) and (IV) ions.
Cerium(III) is amazingly hard to get oxidized to cerium(IV). You need potent oxidizers (e.g. hot persulphate) in order to get it oxidized. Cerium(IV)
oxide, CeO2, even the freshly precipitated oxide/hydroxide only very slowly dissolves in acids, giving a deep yellow solution.
I am afraid that the dissolving of calcined CeO2 will be VERY hard. CeO2 is fairly cheap though.
http://cgi.ebay.nl/CERIUM-OXIDE-Ce-99-8-250g-Very-high-quali...
I have good experience with this seller.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
just a quick question, since quite a few of the rare earth metals share a similar chemistry, would it possible to use Samarium instead?
I have some of that, but the only Cerium I have is in the form of an alloy in Lighter flints.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
yan123
Harmless

Posts: 5
Registered: 4-10-2007
Location: china
Member Is Offline
Mood: No Mood
|
|
generally, CAN is considered as cerium ammonoum nitrate, Chemiscal reviews has a paper talk about CAN (cerium ammonoum nitrate) in 2007.
| Quote: | Originally posted by Xenoid
Oh! No! Please don't use CAN as an abbreviation for this chemical!
CAN is the pretty much standard abbreviation for Calcium-Ammonium Nitrate, a fertilizer which is a blend of about 20% CaCO3 and 80% Ammonium Nitrate.
Wouldn't CeAN or CEAN be more appropriate!
Regards, Xenoid |
i am not for you to cherish.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Xenoid: I think we should always use the proper term in a thread at least once before we use the acronym. I also wouldnt like it if everyone were
throwing around acronyms for chemicals that nobody but experts in a spceific field know about.
I always first explain the acronym (except in obvious cases, like THF or DMF), then I use it for the rest of the thread.
It should also mostly be obvious from the context what CAN would stand for- when people are talking about oxidation of methyl groups or alcohols with
CAN, then one can be sure that it isnt a nitrate fertilizer they are talking about.
| Quote: |
CAN is nearly insoluble in concentrated HNO3, while the REE(III) nitrates are somewhat soluble. Thorium, as the nitrate, would be the main
contaminate, these days thorium doesn't really show up in REE products. |
Thats great to hear, that would be a huge advantage if it is true. But then why does Brauer talk about concentration of the mother liquor to get more
crystals? Maybe the HNO3 isnt so concentrated at that stage of the procedure...
I'll have to see myself.
It would be really nice if you could post the article you were speaking of, but the white, pure CeO2 is the only grade that I can get. Nobody seems to
use impure rare earth compounds any more.
Samarium does not occur in tetravalent form.
The lanthanides that can occur in tetravalent form are Ce and Tb, and to a very limited extent (dont really know what is meant by this- maybe very
unstable?) Pr and Nd.
Of these, only Ce(IV) is metastable in water, the other ones all oxidise water to oxygen and therefore have no aqueous chemistry.
Ce is the most abundant rare earth metal, and by far the easiest one to obtain pure- oxidise it to Ce(IV) and adjust the pH so that hydrated CeO2
precipitates while all other lanthanides stay in solution. Or follow Brauers method with ether extraction of ceric nitrate.
Similar with Eu, which can be reduced to the divalent state and precipitated as the sulfate.
The other rare earths are more difficult to separate.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
A cheap source of lower purity CeO2 is used as a glass polishing material. Mine is pink/salmon color. Try a shop that cuts and installs plate glass,
or their suppliers. Boiling with SO2 solution in water helps SnO2 dissolve in acids, maybe it would have the same effect on CeO2?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Actually it is fairly easy to take Ce(III) to Ce(IV). Simply make a solution of a Ce(III) salt alkalike, and allow it access to air for awhile. The
Ce(OH)3 originally formed will darken to a red-orange, then change to a yellow as increasing amounts if the cerium are oxidised to the 4+ state. To
do it quickly, added H2O2 and then bring to a boil.
Praseodymium also goes into a higher oxidation state, but it forms mixed valence oxides and is difficult to get into any single pure state other than
3+. It may help with the dissolution of ceric oxide by shuttling between oxidation states, which helps do the same to cerium. However the physical
state of the oxide is important too, the low grade oxides used in the older preparations were likely ignited at a fairly low temperature and intended
as a source of the REE rather than as an end product like polishing powder.
I've had a range of difficulty in dissolving CeO2 from various sources. Some would go into solution in constant boiling HCl pretty nicely, others
took prolonged treatment with acid and ethanol, one batch took long boiling with concentrated H2SO4. And one !@#$%^&*( bag wouldn't dissolve in
any acid treatment, I ended up lining a large crucible with a cm or so of the oxide, then filling the center with a mix of the oxide and sodium
carbonate, fusing the mix, and then dissolving that in HCl.
I'll try to get access to a scanner in the morning to get those couple of pages in.
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
Would molten Ammonium nitrate + Cerium (IV) or (III) oxide be sufficient to form the double nitrate salt? I remember there was a discussion here
about using metal carbonates or oxides and ammonium nitrate to produce the corresponding metallic nitrate?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by garage chemist
Samarium does not occur in tetravalent form.
The lanthanides that can occur in tetravalent form are Ce and Tb, and to a very limited extent (dont really know what is meant by this- maybe very
unstable?) Pr and Nd.
Of these, only Ce(IV) is metastable in water, the other ones all oxidise water to oxygen and therefore have no aqueous chemistry.
|
Thank You 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Probably the cleanest way to oxidize Ce(III) to Ce(IV) in solution would be by electrolysis. In the solid state, finely divided Ce2O3 oxidizes to CeO2
on heating in air.
Of the rare-earth metals that exhibit the +4 oxidation state, only Ce(IV) is stable enough to exist in solution, being highly complexed because of
its charge. Apart from CeO2 and salts of oxy-anions, the only other compound of it is CeF4 (and possibly oxyfluorides). The only other rare earths
that stably exhibit the +4 oxidation states are Pr, Tb, and possibly Nd, although only as the non-stoichiometric solid oxides, Pr6O11 and Tb4O7,
obtained by pyrolysis in air of the soluble salts as which they are isolated by ion-exchange chromatography. They are reduced to the +3 state with
evolution of oxygen if dissolved in acids. (It is not certain what the composition of the higher oxide of Nd is, or whether it has been characterized
at all).
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
OK, attacked is the basic prep pages and the cover of the pamphlet I was referring to earlier. I skipped the electrolytic ones, they take platinum
electrodes and are more for analytical grade.
There's a table in there that shows one run where they went for 3 crops of CAN, although the text doesn't mention concentrating the mother liquor. The
first crop was 3/5 or more of the cerium, purity of the CAN was 99,4%. The second crop was less than 1/5 of the Ce with a purity of 98,8%, the third
was smaller at 90% purity. So a second crop may be worth doing, but past that you may be better hitting it with base to recover Ce as the hydroxide.
Under alkaline conditions, H2O2 or air will oxidise Ce(III) to Ce(IV), the resulting Ce(OH)4 can be dissolved in acids to get the ceric salt. This is
as clean as electrolysis, and doesn't take expensive electrodes.
Note that NaOCl bleach will precipitate Ce(OH)4 from a solution of a Ce(III) salt. The hydroxide would need to be washed clean of Na and Cl ions,
although the dissolution in nitric acid will take care of the chloride and a trace of sodium isn't likely to be important in many applications.
Attachment: CAN.zip (579kB)
This file has been downloaded 1358 times
|
|
|
NADPH
Harmless

Posts: 11
Registered: 1-1-2010
Member Is Offline
Mood: No Mood
|
|
I tried to make (NH4)2Ce(NO3)6 all the way from ferrocerium rod.
First I dissolved about one third of huge ferrocerium rod in HCl. Reaction was quite violent (lot more violent than Nd2Fe14B magnets with HCl) and
huge amount of H2 gas and heat was liberated. Also there was strong unpleasant smell. So I decided to seal reaction flask with stopper conected to
rubber tubing to lead the gas far away and out through window (cheap alternative to fume hood). I left reaction overnight and then most of ferrocerium
was dissolved. Then I diluted reaction with distilled water and filtrated off all undissolved particles until solution was clear.
Next I purified Ce(III) by precipitating cerium (III) sulfate with concentrated solution of sodium bisulfate (of course sodium sulfate works as well
but bisulfate was only thing I had at the moment). Greenish Fe(III) was left in solution but white Ce (III) sulfate precipitated. I filtrated cerium
(III) sulfate and washed few times with boilig water. Dried it in oven @120˚C.
Then I converted all the Ce (III) sulfate to Ce (IV) hydroxide by mixing it with hot Na(OH) solution and adding H2O2 to oxidize Ce(III) to Ce(IV).
Before adding H2O2 I cooled reaction in ice bath but anyway H2O2 reacted quite virgously and after I dropwise added few mililiters of H2O2 colour
changed to yellow (fine emulsion of Ce (IV) hydroxide after shaking) but most Ce (III) hydroxide was still unreacted as yellowish white clumps. Since
H2O2 is not cheap, I added bleach (5% NaClO) instead. After few minutes of virgous shaking all clumps transformed to fine yellow emulsion which slowly
settled down. Left ir overnight, filtrated and washed 4 times with distilled water and 2 times with acetone. After drying I got few grams of fine
yellow powder of Ce(OH)4 (hydrate?)
As it was quite expensive to make, first I tried to make ceric ammonium nitrate (CAN) in small scale. I weighted 0.5 grams of Ce(OH)4 (0.0024 mol) and
converted it to CeO2 by heating in test tube with butane torch until no more water was evolved.

After heating I got 0.36 grams of CeO2 (0.0021 mol).

Next I heated stoichiometric amount of ~14M HNO3 (0.9 ml) and then added CeO2. It reacted virgously but lot of CeO2 remained undissolved.

I heated mixture and added more HNO3 dropwise (~0.5ml) until most of CeO2 dissolved.

As a result I got cherry red solution which I transferred to fresh tube to get rid of undissolved particles (after settling them down for few
minutes). Then heated it and added stoichiometric amount of NH4NO3 (0.34 g).

Solutions colour changed from cherry red to orange and orange precipitate formed instantly.

As the colour is exactly as it should be (http://en.wikipedia.org/wiki/File:Ceric_ammonium_nitrate.jpg) I assume orange precipitate is pure (NH4)2Ce(NO3)6
Sorry for my English. I hope you can understand what I wrote.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Wow, very nice of it indeed CAN! This is a nice way to make this material, although it is not THAT expensive when you can buy it (about 1 euro per 6
grams). You are sure that all CeO2 dissolved?
But why did you heat the hydroxide to make the oxide? You had a good chance that the oxide would be unreactive, and hydroxides are generally more
reactive.
|
|
|
NADPH
Harmless

Posts: 11
Registered: 1-1-2010
Member Is Offline
Mood: No Mood
|
|
Almost all CeO2 dissolved. After heating only small pellet of dark brown material remained.
I was not sure if Ce(OH)4 was hydrated or not. An I thought that more CAN will precipitate out if I will get rid of as much water as possible. That is
why I calcined my Ce(OH)4
[Edited on 13-2-2010 by NADPH]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Nice work! just to nitpick though -
| Quote: | | I purified Ce(III) by precipitating cerium (III) sulfate with concentrated solution of sodium bisulfate (of course sodium sulfate works as well
|
Sodium sulfate shouldn't work? If NaOH can convert Ce(SO4)2 to hydroxide then it wouldn't makes sense for Na2SO4 + Ce(Cl)3 to give you cerium sulfate.
I think you need bisulfate or sulfuric acid...
|
|
|
NADPH
Harmless

Posts: 11
Registered: 1-1-2010
Member Is Offline
Mood: No Mood
|
|
bbartlog
Why sodium sulfate shouldn't work? Cerium (III) sulfate is much less soluble than cerium (III) chloride. I think there shouldn't be huge difference if
I use sulfate or bisulfate or sulfuric acid
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Sodium sulfate shouldn't undergo metathesis reaction with cerium chloride. In the case of the bisulfate or sulfuric acid the cerium is displacing the
hydrogen, but it doesn't seem that it should be able to displace sodium.
|
|
|
NADPH
Harmless

Posts: 11
Registered: 1-1-2010
Member Is Offline
Mood: No Mood
|
|
a) NaHSO4 => Na(+) + HSO4(-) <=> Na(+) + H(+) + SO4(2-)
b) Na2SO4 => 2Na(+) + SO4(2-)
___________________
c) Ce(3+)(aq) + SO4(2-)(aq) => Ce2(SO4)3 (s)
I still don't think there will be any difference if I use different sources of SO4(2-) ions
so I will make some Na2SO4 and try to precipitate Ce2(SO4)3.
I am almost sure it will work
_________________
p.s.
sulfate works
I just dissolved some Ce(OH)4 in nitric acid

then reduced Ce (IV) to Ce (III)

and added sodium sulfate solution. White Ce (III) sulfate was formed.

________________________
p.p.s.
I just did slightly different experiment.
weighted stochiometric amounts of Ce(OH)4 (2 g) and NH4NO3 (1.34 g)

dissolved Ce(OH)4 in ~14M nitric acid. Reaction was exotermic with lot of heat an fizzing. Eventually clear orange liquid formed.

Meanwhile I dissolved NH4NO3 in hot water. Then added it to cerium solution.

then poured on small petri dish

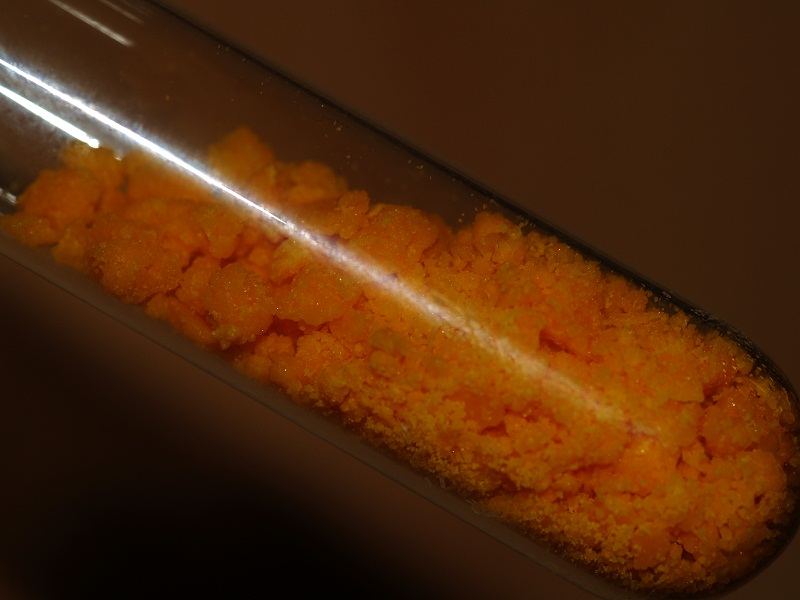
and gently evaporated in oven @120C until solid crust of CAN was formed

when I tried to stir with glass rod, the crust absorbed all remaining liquid and turned in dry hydrate. (According to literature its chemical formula
is (NH4)2Ce(NO3)6*xH2O; x=6-8)

As a result I got 4.4 g of CAN hydrate
[Edited on 14-2-2010 by NADPH]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Pradyot Patnaik
Handbook of Inorganic Chemicals **
McGraw-Hill
2002
Sez ..... electrolytic oxidation of cerous nitrate in nitric acid
to ceric nitrate, followed by the addition of ammonium
nitrate solution.... Or... dissolving cerium (II) oxide ,
CeO-H2O in con. nitric acid followed by treatment with
ammonium nitrate.
** The book was reminded and could have been had
inexpensively a few years ago.
|
|
|
Copenhagen
Harmless

Posts: 13
Registered: 18-6-2010
Member Is Offline
Mood: Lattice vacancies
|
|
I performed an experiment where I tried to obtain Cerium( IV) Sulfate from crude Cerium(IV) Oxide.
Bottger, W. Newer Methods of Volumetric Analysis pg. 27-28
Trial 1
50 g of CeO2 (FW: 172.115 g/mol) were placed into a 600-mL beaker containing 50 mL of 36 N H2SO4 that was pre-heated to 120 C with magnetic stirring.
Addition was slow - scoopfuls at a time using a plastic spatula. Slow addition seemed prudent due to violent bubbling of the sulfuric acid upon
addition of the solid. The careful addition of CeO2 was completed after 1 hour. The solution was heated for an additional 2 hours at 120 C. It was
allowed to cool at room temperature for 30 minutes after which time it was slowly added to a 3000-mL beaker containing 1800-mL of cold water
temperature (3 C). The solution was allowed to settle for 2 days after which time the supernatant was drawn off in portions with a 50-mL Pipet and
transferred into a 250-mL Erlenmeyer flask. The rest of the solution was stored away in the 3000-mL beaker to allow for further settling.
Trial 2
200 g of CeO2 (FW: 172.115 g/mol) were placed into a 600-mL beaker containing 200-mL of 36 N H2SO4 that was pre-heated to 120 C with magnetic
stirring. Addition was slow - scoopfuls at a time using a plastic spatula. Slow addition seemed prudent due to violent bubbling of the sulfuric acid
upon addition of the solid. The careful addition of CeO2 was completed after 82 minutes. The reaction was heated for an additional 2 hours at 120.
After 2 hours it was removed from the heat source and allowed to cool at room temperature for 4 hours. The supernatant was drawn off in portions with
a 50-mL pipet and stored in a separate container. The precipitate was allowed to dry in room air. The precipitate has the bright yellow color
typical of Ce(SO4)2.
Attachment: VolumetricAnalysis.tif (64kB)
This file has been downloaded 1682 times   
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by garage chemist  | The Ce(IV) ion is a powerful oxidiser in aqueous solution, stronger than chlorine, and capable of very interesting reactions, especially in organic
chemistry.
When its oxidising properties are to be used in preparations, it is most commonly employed as the salt ceric ammonium nitrate, or short CAN:
(NH4)2Ce(NO3)6
I think that this reagent has received. |
Uses? Not in my life. I received 5-6 lbs as a gift years ago...
The only use I am aware of is the analysis of the nitrogen content
of azides. And for the destruction/disposal of azides.
Run it through Google.com/books
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Lots of uses for CAN A test for alcohols, which form a red complex then oxidise away. In inorganic and organic quantitative analysis, as an
oxidising titration reagent; less common now due to various instrumental analysis methods. It can cleave the carbon bond in glycols HO-C-C-OH
similarly to lead tetraacetate. A good way to oxidise many aromatic compounds to quinones.
Its oxidation of alcohols, aromatic amines, and phenols, make it useful in TLC for visualising those compounds.
Attachment: ceric_ammonium_nitrate_in_modern_chemical_synthesis.pdf (748kB)
This file has been downloaded 1581 times
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its also used for selective mono-debenzylation of tertiary amines, and for a few oxidations aside what not_important mentions; formation of quinones
rings a bell...
|
|
|
| Pages:
1
2 |