| Pages:
1
2 |
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Simple and inexpensible DIY mini-charcoal "furnace"
note, that I've used the term 'furnace' but that isn't , actually (that's because I don't know the exact term, but you will know as soon as you see)
But the fact is that my friend (from anoher forum) gave a great idea to construct a easy DIY, using only things over the counter and capable of making
a iron thing going to white on heat..
The only things needed are some cans, iron wires and a somewhat thick screen and an old AMD cooler on similair(small fans and the like would work
also)... the problems being in corrosion over the time and in small chunks of charcoal occasionally falling the wires, but this was almost totally
overcome by a screen, and also for regulating the inlet air, a simple object was placed in front of the cooler, to block some of it to going to the
burning BBQ charcoal..
http://www.4shared.com/dir/2601836/293a189c/%3Cb%20style=
the improvised apparatus can be disassembled easily as well (when corrosion eat considerably the cans,etc), but another can be made with the same
cooler just as easy..
Any thoughts on increasing the power of this or improving its resistance?
thanks
[Edited on 24-10-2007 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
This is very interesting and i am highly interested in building such a thing for aluminium melting. However the pictures on the site were helpful,
they didn't give me much on the actual construction function etc. Could you elaborate on the design of such a thing. Only the fan plugs in, the heat
in generated by charcoal correct?
Chemkid
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Well the author of this did this in quite some hours..he only searched some way to contruct such device for the home metal working and the basic idea
"air plus charcoal" comes in their mind.. And all was trial and error.. If you notice, he have used tape (!) to put together the cooler and some epoxi
(!) to put the cans together...and none of these were considerably damaged(!) ..
the basic procedure was taking a larger can, knead a part of it to give a stable bottom as base of the device,opening the part where the cooler will
be placed, cutting a hole in their middle to put the wires/screen and then another (tomato) can (without the bottom) and gluing it with some epoxi..,
after placing the cooler on the right place and the tape..
To ignite , he simple put some kerosene soaked sawdust or paper pieces , the charcoal (with some kerosene also) , strike a safety match , ignite and
turns on the cooler..
Possible and quite obvious improvements being in to use somewhat more benign sealants/cements like gipsum (for the cans) and simple wires to keep the
cooler on the place.. another thing that can be done is instead of cutting the bottom of the smaller can, just letting it in place and making several
holes in it..so you woudn't need screens or anything..
I will do this as soon I get the cooler...
And aside the use in home metal working, this also can be potential to some applications involving catalysts tubes and the like...
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Charcoal furnace
These things can be as simple or as complicated as you want and they almost always work. Beginners almost always overestimate the amount of air
required and then spend an unreasonable amount of effort cutting it back.
Mine is a forge with the main hearth a modified car tyre rim and a 12v blower from a automobile heater fan (easy to adjust the speed and far more air
than necessary at full blast)
It can be as simple as a hole in the ground, filled with charcoal and the side air inlet buried under the charcoal.
I like your idea of the computer fan. Easy to get 2nd hand and easy to adapt to pipe (Tin cans!)
Lovely. Now burn your own charcoal. Just as easy, even in large quantities using a 200 litre drum.
I just love this low tech stuff.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Thank you for the link Aqua, I like the idea very much. A carbon furnace is something I always wanted to have, but it never ocurred to me that it
could be a tabletop device to be used indoors. The simplicity of the design makes it almost disposable, exept for the fan.
Very clever indeed.
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
What about using the cans as a mold, and casting a "L" shape out of concrete? Then dissolve the cans out with some NaOH or just let them burn out in
their own good time. Or alternatively using two pvc pipes with 90degree bends, placed one inside the other and filling the gap with concrete. The pvc
could then be dissolved out with petrol or again just burnt off (that may be bad). By making the horizontal part of the L long, the fan would not need
to be anywhere near the flame and wouldn't be damaged by radiant heat. Just fill with charcoal from the top until it is packed and it will move some
distance along the horizontal part but not all the way. This would allow hotter temps than aluminium melts at and would not burn like iron.
Does anyone know how hot calcium silicate can go before it melts/softens?
Does anyone know how hot a charcoal/air flame can get?
To partially answer my own question, I have once and only once seen a bonfire containing empty beer cans fanned so hard that one of the cans very
suddenly and brightly deflagerated. That places the minimum at ~700*C but I think it was hotter. Actually I am sure, it was BRIGHT orange, probably
over 1000*C.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
| Quote: | Originally posted by ciscosdad:
Mine is a forge with the main hearth a modified car tyre rim and a 12v blower from a automobile heater fan (easy to adjust the speed and far more air
than necessary at full blast)
It can be as simple as a hole in the ground, filled with charcoal and the side air inlet buried under the charcoal.
|
Great! What you was capable of melting/casting with that? A hole in the ground and a side pipe leading air to the charcoal is a lovely idea also! Easy
to made in the backyard and good to hold greater amounts of things to be melted,etc..
Some people here were talking about alternatives to the cooler..Some people even have used a hair drier to do the job..I'm surely which doing this
isn't very much interesting , because of power wasting and also to be less lasting and restricted of running at lesser times, but maybe it will give
greater temps, because of the inlet of hot air.. what about?
| Quote: | Originally posted by Tacho:
Thank you for the link Aqua, I like the idea very much. A carbon furnace is something I always wanted to have, but it never ocurred to me that it
could be a tabletop device to be used indoors. The simplicity of the design makes it almost disposable, exept for the fan.
Very clever indeed.
|
Thanks. Yes , this never ocurred to me too.
And probably this desing can be easily upscaled without much trouble.. Maybe a larger (18L) paint can with the charcoal and in the lower part several
tubes or biggers cans (i.e. 900mL soybean oil can) attached to it , and each of these holding a cooler , or alternativelly , one can probably use one
tube with a bigger fan attached.. Maybe using several PC coolers can be better , since the air is minutely feed to the burning charcoal..
And for this I agree with the idea of Antwain in protecting the forge , because using this larger device , is less probably to use it as a
'disposable' forge, and this one can probably be easily protected with some sort of cement/refractory(best).. But this wouldn't look as a 'tabletop'
device 
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Antwain
...
Does anyone know how hot calcium silicate can go before it melts/softens?
Does anyone know how hot a charcoal/air flame can get?
... |
http://books.google.com/books?id=Q11iM9FXDqQC&pg=PA89&am...
1544 C Lange's handbook has several pages of ceramics related data, one from the 1930s to 1960s is just fine.
Charcoal fires can reach 1100 C or thereabouts, but that takes some care to reach.
Note that there are other limits to ceramics other than the melting point of the pure substance. Phase changes may cause the solid to break up, the
ash can for lower melting point mixtures with the original ceramic.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Dave Gingery Charcoal Foundry
Google " Dave-Gingery charcoal" for other designs inspired by this one.
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Furnace design
I like Antwain's idea about casting concrete as the air passage, but be careful of the concrete's response to heat. It can deflagrate (from included
water) if you're not careful. I guess a (disposable) can at the hot end would solve the problem.
I believe you can melt Cast Iron with a charcoal or coke furnace, but not pure or wrought iron. It appears as though this is the limit of its ability
though, as it takes hours to get the charge melted. 1100C sounds a bit low, but I'm not certain of the exact capablilty. The blacksmithing pages will
give you an idea.
If you want the upper limit, you will need a purpose built hearth with firebrick and proper insulation. I won't even guess how much the crucible will
cost you. However anything from Al to the bronzes should be easily within reach. Melting copper would probably be as high as I would arttempt with
mine (1080 Deg C ?)
I have seen a backyard built forge consisting of a hole in the ground, a vacuum cleaner with a bit of steel pipe and 2 bricks (to hold the crucible).
Charcoal was simply shovelled into a pile as big as necessary to keep the crucible more or less immersed. He was melting Aluminum in a piece of steel
pipe (bottom welded shut of course). Air flow adjustment was moving the Vacuum cleaner hose closer to or further from the end of the buried steel air
pipe. Full airflow would blast charcoal all over the place.
A good insulator for the lower end of the spectrum is Plaster of Paris. Suitably dried out of course. I believe it is good for 1000 C or so. I used it
to line the bottom of my forge (the Auto rim!). Just in case, I keep a layer of ash on top of it (couple of centimeters) but that's probably not
really necessary for what I use it for (occasional blacksmithing). Plaster may actually be a good substitute for Antwain's concrete. Just dry it
thoroughly first.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
ciscosdad , Antwain, not_important and Eclectic : thank you guys very much for providing these links and ideas.. (I wil check out also N.I.'s hint for
Lange's datas on ceramics/refractories)
I'm also highly interested in home metal casting/melting and also in some other applications of forges anf furnaces, that's simply because reaches
good temps (at least for me, just beginning in this business) and also is much more cheap (oxy-acetylene torchs are somewhat expensible here, as is
MAPP gas devices also), and of course is a sweet homemade thing.
@ciscosdad , I'm quite sure : if the forge is treated with care and some sort of improvement is made, this one can melt copper or maybe even more..
Someone on the another forum stated which was able to melt aluminium and also copper using an old big cast iron truck wheel in the ground and some
sort of mechanism in which a 12v auto blower was attached and blowing air in a larger tube leading to bottom of the wheel ..
this know site is one good example to get some resumed info :
http://www.backyardmetalcasting.com/
And now comes to my mind another doubt, which maybe can improve even more the desing of such device:
in the above site, he said that hardwood charcoal is hotter/better than BBQ charcoal. Is that sure? Has anyone some sort of data/info about this
issue? (Also) What the best wood for home furnace applications?
And for upscaled charcoal making in backyard , someone can build such device:
http://64.176.180.203/charcoalretort.htm
seems to be somewhat expensible, but someone very interested can make it with some effort.. or maybe, the old technique of producing charcoal, burning
the wood in a clay based shell and let burning with small amounts of air... 'charcoal burned to death' or something similair
[Edited on 25-10-2007 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Wood
I believe that hardwood is best for heating charcoal, but I have had very limited experience, so someone else may have better info. Brazilian timbers
are an unknown to me.
The timber I prefer is Jarrah ( an Australian hardwood of density about 0.7). I have had another timber (Wandoo) recommended as especially hot and
dense for hot fires, but I have not tried it. The timber is denser than water when dry (sinks!). It also makes a lot of ash. Much more than Jarrah.
I guess the idea is that a nice dense wood will make denser charcoal than a softer wood. However, it is probably not critical for the low demands we
are likely to make of the process.
Barbeque charcoal usually has binders added to make the little briquettes (clay or whatever) and is not as good as natural charcoal. You may be able
to collect enough charcoal from local scrub after a bushfire in the right season. That may give you enough of a variety to experiment a little if
required.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Have none of you been to this site: http://www.backyardmetalcasting.com ? There is a forum there with some very good information on making very useful furnaces, forges, and kilns,
along with information on a variety of foundry and craft related topics. Check it out! Much of what we cover in this thread, and others on SMDB has
been examined in great detail.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Yes we have seen that one (see a couple of posts above), but the emphasis here seems to be small and simple, more a benchtop device than the much more
ambitious ones in that forum.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
@ciscosdad , there are several kinds of wood here (look at http://www.ibama.gov.br/lpf/madeira/foreword.htm )
But as you emphasised, 'keep it simple' 
the most common timbers here are
Brazilian Cherry
Willow
Plum (very low density.. charcoal of this will do one of the best black powders..said better even than willow)
acacia
balsa
bamboo
Cottonwood
Lime
Pine
Umbauba
(the woods above are *mostly* low density) but there are MANY others, but I dont know the english name of none.. very hardwood being i.e.
Roxinho,Imbúia , Braúna ,Pau-ferro and lots of others..
BTW ciscosdad, most brands of BBQ charcoals have also, furthermore , other chemical substances than binders (some NaNO3(only thing that seems somewhat
benign to our application  ), lime ,borax,etc) and some even have some sort of
coal in it (anthracite,etc). This without speaking in ash content which is almost always very high. ), lime ,borax,etc) and some even have some sort of
coal in it (anthracite,etc). This without speaking in ash content which is almost always very high.
But again in wood issue, I know that generally,some of the high density woods are used in common OTC BBQ charcoal sold, because they tend to burn more
slowly than the lighter ones. but what I can do to get high density wood(what the best procedure to charring it, 'tricks', etc)?
And also, is know that these high d. woods generally have greater resin (and other things) content. Is that good to a forge or furnace, or this need
to be removed in charring process?
thanks
@Fleaker ,thanks. The guy on this site (backyardmetalcasting.com) have an excellent design of a kitchen/car oil based furnace, which besides reaching
high temps, is more complicated to did and requires some time/$ to do..but surely is still great and possibly a 2nd choice or a better design to
people that know the art , and/or do not want more BBQ charcoal lying around..
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hardwood charcoal burns the longest and hottest. Coal is a short second, high in density but because it's dense, tends to burn cooler. (A lot of
surface area, like the coke in a blast furnace (which is also well insulated, by design and by scale!) will easily burn up around 1700C, leaving
molten iron and slag.)
There isn't anything special you can do with soft woods. Burn them as-is for general heating purposes. Don't bother making charcoal with it unless
you want to make BP  or distill the voltatiles (methanol, etc., resin, tar,
...). You won't get as high an ultimate temperature because it takes heat to burn it out before it burns up. or distill the voltatiles (methanol, etc., resin, tar,
...). You won't get as high an ultimate temperature because it takes heat to burn it out before it burns up.
Though I will mention that I once stoked a fire of white oak hunks (an excellent hardwood) up to yellow-white heat with a nice blower. Just because
volatiles are burning out doesn't mean you can't brute-force it hot... it's just not efficient. One interesting aside about such a fire: because it's
burning so hellishly hot, the exhaust smells *perfectly clean*, only the warm moisture of hydrogen oxide and the sensation of breathing "spent air",
carbon dioxide.
My website (which leads to ABYMC under the metalworking section) has a bunch of stuff on the subject, too.
Tim
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
The 'primitive' way that I'm refering earlier to charring charcoal is the charcoal pit(earth kiln)..
from the Black Powder Manufacturing, Testing & Optimizing about charcoal:
| Quote: | In the smallest pits charcoal is made by starting a fire at the bottom of the pit and then adding
wood until the pit is full. This wood is then covered with leaves, grass and finally with earth.
The carbonization takes about two days.
Larger pits are first filled with wood and then fired in the center, where an open space going
right to the bottom of the pit is filled with combustible material. The wood is then allowed to
burn well before it is covered with vegetation and earth. Air vents are made in the covering to
control carbonization. Adjustments to the carbonization rate are made by opening or closing
these air vents. The whole cycle, including cooling can take up to a week.
The largest pits have an air inlet on one side and a smoke outlet on the other. The bottom layer
of wood is loosely piled lengthwise to allow air flow from one end to the other. Alternate layers
of crosswise and lengthwise wood are piled on top of this first layer. Unlike the first layer, these
layers are densely packed. The wood pile is then sealed with vegetation and earth. The fire is
started at the inlet side, and slowly burns its way to the outlet.
Carbonization may take up to a whole month in the largest pits and cooling a month or longer.
Charcoal pits can be very dangerous as persons may step on them, not realizing that there is
hot material under their feet. The most dangerous time is during the cooling cycle when no
smoke or vapors are given off, thus giving no warning.
While charcoal pits are easy to construct they suffer from the following disadvantages:
• Carbonization is difficult to control.
• The charcoal yield is low.
• Pits are not suited for very small scale production.
• Pits cannot be used during the rainy season.
• Charcoal may end up being contaminated with other matter such as earth. |
Well, this all seems to be simple to do in the backyard than making a charcoal mound,being the simplicity of such procedure the most attractive to the
amateur although many disadvantages (well, this can probably differ somewhat, because the people using such procedure can make your own charcoal, but
may not use it for BP's making)
Hey Tim, besides the high density and greater amount of carbon , can the cooler temps of coal be influenced somewhat by 'volatile' contents, or this
is just wrong?
lol, your page about melting and related items http://www.abymc.com/tmoranwms/Metal_Casting.html is excellent!
excellent too is
http://www.abymc.com/tmoranwms/Casting_Charcoal.html
http://www.abymc.com/tmoranwms/Casting_CharBrass.html
Thank you very much for these links
[Edited on 26-10-2007 by Aqua_Fortis_100%]
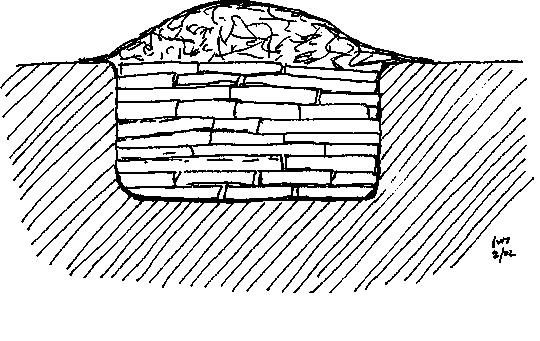
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
The resin content and any other characteristics of the wood may or may not affect the charcoal. The only way to be sure is to experiment. I have made
charcoal from Jarrah and Pine, (the extent of my experience) and since the Jarrah does what I want, I have not explored further.
I recommend the hardwood if you can get it, but I note that there are a lot of guys using pine, including the one with the 2 barrel charcoal cooker.
There may just not be that much difference. OTOH you could design some experiments with Brazilian woods to determine which is best and report back
 . First decide your criteria though. Temperature? Physical characteristics of the
charcoal? Ash content? Flame characteristics? . First decide your criteria though. Temperature? Physical characteristics of the
charcoal? Ash content? Flame characteristics?
My guess is that you will find your locally most common and cheapest available wood and go with that. Start small and simple so you can get a feel
for the process and what it can do for you.
Best of all: Take some photos and report back to us! 
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I truly doubt that making your own charcoal is worth the trouble, unless you think of it as an interesting experiment itself.
I have never done it myself, but all the information available seems to show that is a very messy process that involves lots of fumes, tar sticking
everywhere, and a lot of dirt to clean afterward. Not to mention that, like every art, it may take practice to obtain good results.
I simply do not believe that the small difference in final temperature (if any) can overcome the simplicity and cheapness of just buying a bag of
barbacue charcoal.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Making Charcoal
From Lindsay Books, a great resource for the mad scientist. 
They have a few other books on making charcoal, and LOTS of stuff on home foundry work.
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
@ ciscodad- do you really think that plaster of paris could go that hot? i thought it dehydrated above ~400*C and basically turned to powder...
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
@ciscosdad , thanks you by the reply. Unfortunately I still haven't my own cam, so is somewhat difficult. Furthermore, here is coming the raining
season , so even if I could have the cam, I still would be unable to do the charring procedure(I certainly don't want to do the procedure indoors on
even in the garage). But I will do this latter and will post here, even if I will use low density wood (that's the most commom) for BP.
The hardwood is somewhat difficult to get, but is still easy.. In my backyard is growing a BIG pine tree , so I can in near future make something
interesting with it..
@Tacho, yes, this seems to be really a messy process, but this would be just for fun.. Most people that have done it, were highly happy with the
results (for BBQ , BP or another application)..and some people even get a more funny procedure because they have used charcoal(BBQ) as heat source to
make their own charcoal!!!
@Eclectic, these Lindsay Books, were very helpful(specially the process of making both charcoal and energy), thank you for linking it..Now they are
all in the proper place: my favourites list 
@Antwain , I'm also worried about gypsum.. That's because is one of the most OTC here..but think you that anything can be made to improve its
resistance? maybe putting some portland or another kind of cement (the grayish stuff from hardware stores) instead..(?)
[Edited on 26-10-2007 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
PoP retains its structure to absurd temperatures, but obviously loses a lot of strength in the process. In particular, over 1100C it loses sulfur
(poison for any metal you're pouring into the mold) and shrinks as it decomposes to lime.
Re: coal: it burns just fine, once you get it burning. Of note, due to its density, it can be hard to ignite. A typical coal forge starts with about
1/2" (1.25 cm) screened coal, ignited with lighter fluid and wadded newspaper. Alternately, a fire stoked from wood and charcoal, which are also easy
to ignite and hard to snuff out, would work. When you're forging, what you want to do is stoke up a good burn in the center, and let the volatiles
cook off (yellow flame). A whispy blue to red flame, from just carbon monoxide burning, is what you want. As you burn up the coke, you'll need to
push clinker out of the way and rake in new coke, which has been made from the coal around the side of your forge burning away in the mean time.
Tim
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Thank you Tim, was very helpful.
Very good note about sulfur..I didn't know about this. (only that I've know was this mineral coal that have some in it) .Probably a small detail in a
home furnace or forge design , but really important to metal melting/casting. What the metals (both from the crucible, forge and the target metal to
melting) will be damaged considerably with sulfur present in coal? The old S.C. Wack's mellor book states that only platinum and few other elements
will not bond to sulfur..that's seem to be a bad news, although SO2 evolved seems to be of little danger (except for the natural nauseous smell and
toxicity)..
Backing on the subject, what the best OTC or homemade refractory to make a forge/furnace?
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
I may be pushing towards a slightly different goal here, as I would happily trade a bit of 'smallness' and simplicity for the ability to reuse it
continuously. I don't have room for a benchtop one anyway, ie. it would take up half of my bench, so if it was double the size this wouldn't worry me.
What about making an L design as I suggested earlier out of calcium silicate cement, then coating the inside of it with some waterglass before use.
This may melt and/or seep into the cement, and/or dissolve/mix with some of the carbon, but I don't see this as a problem.
calcium silicate has a high mp. (not sure what exactly). SiO2 is ~1600*C and sodium silicate is ~1000*C. I don;t see this as a problem, since it
should seep into (or dissolve and make a eutectic with) the calcium silicate. This would provide a high mp. glassy covering to the inside of the
combustion vessel. Further sodium silicate could be added after several uses of the device, or after every one, to prevent damage to the inside of the
vessel. If necessary, the inside surface could be scrubbed with something prior to each application of waterglass.
|
|
|
| Pages:
1
2 |