| Pages:
1
2
3 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
SO3 from Iron Sulphate
The oldest process for preparation of oleum is the destructive distillation of FeSO4.7H2O (green vitriol, copperas).
Merck says this loses water at 300 C and decomposes at higher temperatures. I suspect this goes:
FeSO4 -> FeO + SO3
If so about half the mass is converted to SO3.
Might this be the basis of a practical lab scale preparation of SO3 or oleum in a tube furnace?
No catalyst required
No SO2 feed required
Relatively modest temperatures (maybe) compared to vanadium process
My tube furnace is 12" x 2" and good for 1100 C which is a lot more than required. If this decomposition will proceed at pyrex temperatures that would
be very convenient.
Comments and opinions?
[Edited on 4-9-2007 by Sauron]
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
When I was young I made nitric acid by a rather old method, which involved heating potassium nitrate with ferrous sulphate and (if I remember
correctly) alum. Not sure what the alum was for. I was only about seven or eight and just doing it in test-tube quantities, but it did work,
presumably due to the liberation of sulphur trioxide from the ferrous sulphate.
Pyrex test tubes were fine at the temperatures involved (I heated over a gas flame), but they were rather difficult to clean afterwards....
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If what remains in the tube is Fe) (ferrous oxide) then acid ought to dissolve it out. In any case, a two or three foor length of 2" pyrex tube is
cheap enough to be disposible. If the informal calculations I have made in my head are correct than 30 cm x (2.5 cm) squared x pi x 1.8 (density of
ferrous sulfate) should be the mass of the charge and about half of that should be SO3 product or about 500 g SO3 theoretically, which ought to make
several liters of c.25% oleum, worth a lot more than the frigging pyrex tube.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
The old way for forming SO3 for the production of fuming sulphuric acid was heating (dry distillation) ferric suphate, not ferrous sulphate!
Fe2(SO4)3 ---> Fe2O3 + 3SO3
Heating ferrous sulphate produces sulphuric acid.
However, ferric sulphate is quite easily prepared from ferrous sulphate.
2FeSO4 + H2SO4 + H2O2 ---> Fe2(SO4)3 + 2H2O
The ferric sulphate crystallises with 9 water molecules and has to be dehydrated first.
But you are right! It does seem to be a simple route for the amateur, no sulphur burners or catalysts!
Regards, Xenoid
[Edited on 2-9-2007 by Xenoid]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The sources I read specified green vitriol and that is ferrous sulphate monohydrate, not ferric sulphate, sorry. They were quite specific about green
vitriol.
"Originally prepared by heating alum, green vitriol and other sulphates, and condensing the products of distillation, sulphuric acid, or at least an
impure substance containing more or less sulphur trioxide dissolved in water, received considerable attention at the hands of the alchemists. The acid
so obtained from ferrous sulphate (green vitriol) fumes strongly in moist air, hence its name "fuming sulphuric acid"; another name for the same
product is "Nordhausen sulphuric acid," on account of the long-continued practice of this process at Nordhausen. " Encyclpedia Britannica 1911
So product is oleum not just H2SO4 but SO3 in H2SO4, which is what I am after. SO3 can always be distilled out of oleum if needed.
It is possible of course that both ferrous and ferric sulphates may undergo this decomposition.
Anyway, do you have any details as to the decomposition temperature of ferric sulphate?
I did manage to look up the mp of FeO, it is 1360 C. So the ferrous sulphate ought to leave black FeO powder behind.
[Edited on 2-9-2007 by Sauron]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I remember ferrous sulfate decomposing in steps, first to a basic sulfate and sulfur dioxide, then the basic sulfate to the oxide and sulfur trioxide.
Ferric sulfate decomposes to the trioxide and oxide at 460 to 500 C, which puts it in the range of the vanadium contact process.
I think ferrous sulfate decomposes somewhat lower than that, starting as low as 380 C. The exact temperature and decomposition products may depend on
the actual starting material, 7H2O, 4H2O, or monohydrate, and the heating rate; as well as the amount of air in the system, the iron oxide acting as
a catalyst to form SO3 from SO2 and O2.
Older chem books (mid to late 1800s) mention heating either Fe(II) or Fe(III) sulfate as a method of generating SO3 in the lab.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
This is all hashed in the SO3/H2SO4 thread, Sauron.
|
|
|
Jamjar
Harmless

Posts: 18
Registered: 2-7-2007
Member Is Offline
Mood: No Mood
|
|
ferrous sulfate decomp
5730950 Sulfuric acid waste recycling by regenerative process
"Thermal performance data for retorting of ferrous sulfate crystals are in FIG. 2, showing evolution of water of hydration up to about 130.degree. C.
and sulfuric acid and sulfur trioxide evolution 14 and 15 at about 680.degree.-750.degree. C. with differential thermal analysis and thermal
gravimetric analysis results for heat release and mass change for ferrous sulfate hexahydrate. Undesirable sulfur dioxide production 16 occurs above
750.degree. C. shown in FIG. 3."
"Scale up testing to date for the pilot process indicates that temperature control in the roasting mass of ferrous sulfate is critical. This
temperature must not exceed 750.degree. C. At 1000.degree. C. sulfuric acid equilibrium favors sulfur trioxide. At 1100.degree. C. sulfur trioxide
decomposition equilibrium favors oxygen and sulfur dioxide."
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, @jamjar. I will have a look at that patent.
@12AX7, which thread? There are many regarding SO3/H2SO4 and the search engine is really not very helpful.
I read gc's persulfate thread (excellent) and the excellent thread on V2O5 catalysis but saw nothing about iron sulfates,
Anyway I think SO3/oleum is of general interest and a practical bench scale method is not a waste of space.
-------------------
Merck Index does not shed any light on the decomposition of Ferric sulfate.
Ferrous sulfate heptahydrate drops to tetrahydrate at about 56 C and monohydrate at 65 C.
Other sources say that the monohydrate loses water at 300 C. The patent information posted by jamjar indicates the decomposition of FeSO4 to SO3 and
FeO occurs between 680 and 750 C and that higher temperatures are to be avoided.
Xenoid's information is that one mol ferric sulfate gives three mols SO3 and one mol Fe2O3. Ferric sulfate is extremely hygroscopic so, must be dried
thoroughly first.
In order for FeSO4 to produce oleum rather than SO3 (without using H2SO4 as the receiving solvent) water of hydration must be condensed, so assuming
that the monohydrate loses that last H2O at <680 C, then FeSO4 would give only SO3. (leaving FeO behind.)
I need more information. I think I will have a look in Ullmann and in Kirk-Othmer and see what they have to say.
[Edited on 3-9-2007 by Sauron]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=727
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, but, no one in that thread seems to have been much into citing references. The thread author (Organikum) was talking about 480 C for complete
decomposition of ferrous sulfate while Axehandle (who seems to have absented himself since this 2003 thread) said 480 was decomposition temperature
for ferric sulfate. Another poster warned that any oxygen from air in the reactor would oxidize FeSO4 to Fe2(SO4)3 at these heats, but apparently he
was unaware that ferric sulfate would also decompose to SO3 (or else he would not have been so concerned.)
As Organikum is probably disinclined to be helpful and Axehandle is not around, I am left to find my own way so I am unashamed to have started my own
thread.
Rest assured that I will document whatever sources of information I come up with. Anything less is chaos and confusion. That old thread veered off
onto numerous tangents, mostly unproductively. I'd like to keep thos one on topic: the decomposition of iron sulfates (ferric or ferrous) to SO3, or
oleum - I am not interested in H2SO4 per se.
I admire the work of garage_chemist with persulfate, but it does not lend itself to even modest scaleup.
Likewise I admire Fleaker's work with a bench scale contact process, I am just looking for more simplicity.
[Edited on 3-9-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
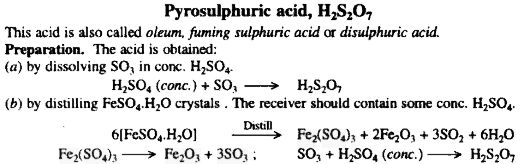
Jamjar was kind enough to furnish some references:
http://sciencemadness.org/talk/viewthread.php?tid=1889&p...
S.C. Wack said
"As for the ferrous sulfate, oxidation to ferric is not a problem, according to Mellor. He says that heating FeSO4.H2O proceeds through the ferric
intermediate.The very first prep mentioned of SO3 by him is: Fe2(SO4)3 + distill = Fe2O3 + 3SO3"
Advanced Inorganic Chemistry By Satya Prakash
http://books.google.com/books?id=LmiVsfw46qwC&pg=RA1-PA1...
[Edited on 3-9-2007 by Sauron]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Sorry, Sauron. That reaction was in my Mellors also, not sure how I missed it!
Perhaps the bisulphate - pyrosulphate is an easier route!
Regards, Xenoid
[Edited on 3-9-2007 by Xenoid]

|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The author in Mellor was probably just trying to keep it simple, it was already long known that a basic sulfate was an intermediate from the
monohydrate. Some recent investigation into this (and with a basic sulfate identified under the conditions used) and also the differences in heating
the different sulfates of iron and other metals can be found (in German of course) here.
[Edited on 3-9-2007 by S.C. Wack]
Attachment: metal_sulfate_decomposition.pdf (1.1MB)
This file has been downloaded 2345 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, that is indeed an illuminating document.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The mass/temperature curve for FeSO4 is found on page 41 and that for Fe2(SO4)3 on page 45.
Most interesting is the statement that the decomposition of aluminium sulfate is complete to 80% already at 500°C! This might be a more worthwhile
material for experiments than iron sulfates.
Al sulfate loses its crystal water at 130°C and starts giving off SO3 at 350°C.
However, the decomposition only proceeds to a material of the presumed formula Al5O2(SO4)3 whose decomposition to Al2O3 only takes place at 900°C,
at which the SO3 almost completely decomposes to SO2 and O2.
Ferric sulfate is the other material that gives good yields of SO3 at comparably low temperatures. Its decomposition is complete at 600-700°C and
does not, like ferrous sulfate, give off SO2 first by reduction of half of the sulfate ions.
So turn your FeSO4 into ferric sulfate with H2O2 and H2SO4 and you will be able to get two times as much SO3 out of a given amount of FeSO4.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ferric sulfate hydrate is purchasable (Alfa Aesar).
Thanks for the comments, fellows.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
This is the wrong compound, but it's easily remedied as garage chemist mentioned.
FeSO4
270161642946 is the item number on http://www.ebay.com
3.50 USD/pound.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
From an agricultural supply, it's about $20 for a 50 lb bag. 
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@Sauron - very interesting thread. Do you know the practical temperature limit for a Pyrex tube? I assime it's well below the softening temp.
@S.C. Wack - good reference, will add it to my pile. Pity it's in German, slows me down a bit. It's nice to see a PhD thesis that isn't all useless
obfuscation - same goes for patents!
Regards, Der Alte
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The manufacturer's say 500 C is max short term working temperature for Pyrex etc., 550 is annealing point, I would think that 450 for continuous duty
is likely a reasonable number.
Obviously, therefore, Pyrex and similar borosilicate glassware is not the way to go for this process.
Vycor with its 900 C limit would be ideal, as the process temperature for ferric sulfate decomposition is <700 C.
SS or Monel would also be just fine.
The engineering of the receiving end, is something Fleaker is working out as his SO3 from contact process is exiting at about same temperature. So the
first priority is resistance to hot angry SO3, and the second is avoidance of blockage from SO3 solidifying downstream.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Yes, and from what I've seen, go with stainless 316. I honestly haven't had a single problem with it! After cooling down, my examination of the inside
showed a somewhat bright, reflective interior. I have not run the operation all day and all night at high temperature, but for an hour's work, I would
say that stainless is resistant to it.
Quartz and Monel are wallet-busters; even SS316 hurts if it's the flanged stuff (which I used because it's gas tight).
I'm with Sauron: pyrex is a no-go on this. Personally, if I were to do it, I would look for a stainless drum of some sort, some stainless piping,
insulation, and a very decent welder. In my mind I am picturing an apparatus somewhat akin to what ordenblitz did for his benzene (decarboxylation of
sodium benzoate) still. Seek it in member publications if you've not already looked at it.
If I can find such a piece of equipment, I will definitely give it a shot. While it's nice to have the contact process, it's a bother to set up. Just
piling up a bunch of charcoal and heating 10 kgs of ferric sulfate is much simpler--no flow rates, no residence time worries, no hot zone/cool zone
concerns, just simplicity.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Two inch 316 seamless 18 inches long to fir my tube furnace, seamless tubing maybe 16 gauge wall (1.5 mm) welded closed with a 316 plug at one end -
the plug fitted with an inlet for carrier gas. Other end similarly closed but, with smaller diameter 316 tubing (also seamless) long enough to serve
as an air condenser capable of bringing the SO3 down to something like 200 C.
Then a cooling system to remove more heat, to bring the SO3 down to the lower end of its vapor phase, so that solidification is avoided.
Finally, either dissolution in H2SO4 loop or, a series of traps to condense SO3 per se.
I'll be happy to follow Fleaker's lead as he is well ahead of me in practice.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I was just considering the Stoichiometry of the partial decomposition of Al2(SO4)3 to Al5)2(SO4)3 and SO3
That must be 5 Al2(SO4)3 -> 2 Al5O2(SO4)3 + 9 SO3
I believe that is balanced.
So while Fe2(SO4)3 is more efficient on a molar basis, aluminum sulfate might well be competitive on a weight basis - how many mols per Kg? Here the
aluminum compound has a slight advantage but, it is utterly begated by the density issue - how many mols can we put in a given volume of reactor?
Ferric sulfate, anhydrous, d 3.1
Aluminum sulfate, anhydrous, d 1.61.
So ferric sulfate wins hands down. More SO3 produced per mol, more mols per volume by far and almost as many mols per Kg.
So now let's go look for a published procedure for converting the ferrous sulfate (so readily available) to ferric sulfate (available but not nearly
so readily.).
First stop: Brauser.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by Sauron
So now let's go look for a published procedure for converting the ferrous sulfate (so readily available) to ferric sulfate (available but not nearly
so readily.).
|
Check out my first post, it's from Mellor!
However, ferric sulphate is quite easily prepared from ferrous sulphate. Nitric acid can be used in place of H2O2.
2FeSO4 + H2SO4 + H2O2 ---> Fe2(SO4)3 + 2H2O
Boil the "ingredients" until the solution ceases to give a blue ppt. with K-ferricyanide.
On concentration the solution deposits a whitish mass;
Fe2(SO4)3.9H2O.
The anhydrous salt can be obtained by heating the hydrate.
Regards, Xenoid
|
|
|
| Pages:
1
2
3 |