DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
A how to question
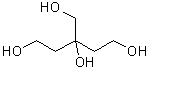
How to synthesize 3-hyrdroxymethyl-pentane-1,3,5-triol?
My guess is it can be prepared by some aldol reactions in basic enviorment, but I need exact method.
Heaven is a half-pipe
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Go look at the structure of citric acid, bring along a bunch of hydrogen.
|
|
|
DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
Yes reducing citric acid is one possible way, but I'm looking for a cheaper reaction.
+ with LiAlH4 I'd have to protect the OH with AcOH and add EtOH to all 3 -COOH-s.
[Edited on 2-7-2007 by DDoS]
Heaven is a half-pipe
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by DDoS
Yes reducing citric acid is one possible way, but I'm looking for a cheaper reaction.
+ with LiAlH4 I'd have to protect the OH with AcOH and add EtOH to all 3 -COOH-s.
|
This doesn't make any sense. Why would you want to protect a group that you want to reduce anyway? Not to mention that the esterification with EtOH
would do nothing to protect the carboxylic groups toward LiAlH4. Both, O-acetyl triethyl citrate and citric acid get reduced to the same compound,
namely the one you are looking the synthesis for.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
1) Better yield
2) Reducing without blocked OH will cause it leaving as water and formation of a double bond instead of it.
Heaven is a half-pipe
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't get it. Why would it lead to better yield? Why the ridiculous amount of superfluous work in order to esterify citric acid and then acetylate
it? Does it sound simpler than to prepare anhydrous citric acid from its monohydrate and then just reduce it?
I would understand if you would have some safety concerns due to the danger of adding LiAlH4 to citric acid (argon is extremely advisable!) but your
arguments make no sense. For example, how is H2O supposed to eliminate from citric acid during the reduction? The carboxylic groups immediately
deprotonate hence making the E1cB elimination (the only allowed mechanism under such conditions) extremely difficult, if not completely impossible
since the alcohol group, once itself deprotonated as well, can not leave. On the other side, with carboxyl groups esterified and alcohol group
acetylated you create just the perfect conditions for such an elimination: you maintain the electron withdraving properties of the carbonyl groups
even in the highly basic ambient making the alpha-deprotonation required for E1cB elimination possible and furthermore now you have a better leaving
group (the -OAc) at the beta position that is, unlike the alcohol, unable to be blocked by deprotonation.
It might be possible that for some reason the yields would be lower, but you provide no reference but only some speculation that furthermore makes no
sense mechanistically.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The protons protonate some of the LiAlH4, creating useless hydrogen and wasting LiAlH4. It is common practice to esterify carboxylic acids before
reducing them with LiAlH4 due to this reason.
Ever added ethyl acetate to LiAlH4 solution? There is no hydrogen being produced, contrary to acetic acid.
|
|
|
DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
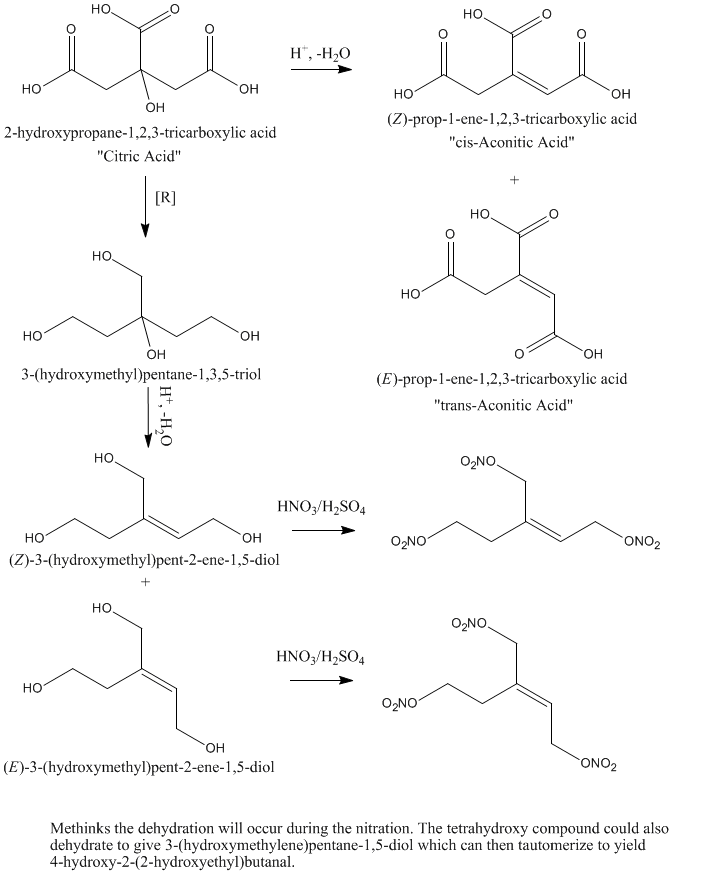
Back to the topic- I need a method how to synthesize this tetraol with some aldol reactions in basic enviorment followed by Cannizzaro reaction. Like
preparation of pentaerythritol.
And yes, blocking with AcOH might be a bad idea. (In matter of fact I have neutralized LiAlH4 with AcOEt),
Some quotes from another topic.
| Quote: | Originally posted by vulture
You're going to waste ALOT of LiAlH4 if you're stupid enough to reduce citric acid directly. Not to mention the danger involved.
|
Oh, and O3 posted this image before in another topic.
Look only like the first reaction
[Edited on 3-7-2007 by DDoS]
[Edited on 3-7-2007 by DDoS]
Heaven is a half-pipe
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
LAH, DIBAL-H, and a number of other hydrides will reduce RCO2(-) to RCH2OH. So you take citric acid, react it with sodium or lithium carbonate, plop
that in EtOH/toluene and add an equal molar amount of NaOH or LiOH, remove water as the azeotrope thus converting the OH to O(-)M(+). Remove the
toluene and excess alcohol, take up the salt in the proper ether, add LAH, none of which gets wasted on acidic hydrogens.
Note that acetylating the hydroxy is going to waste more LAH than just leaving it bare
4 ROH + LiAlH4 => 4 H2 + ROLi + (RO)3Al
RO-COCH3 + LiAlH4 => RO(-) + EtO(-) + lithium-aluminium-oxytrash
Catalytic hydrogenation can be used to reduce the tri-ester, as a metal hydride free route.
I don't see the loss of water under alkaline conditions.
The aldol-like routes I thought of all had too many steps, didn't seem worth bothering with.
|
|
|
DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
| Quote: | Originally posted by not_important
The aldol-like routes I thought of all had too many steps, didn't seem worth bothering with. |
Well I need cheapest way to prepare this tetraol. All thoughts are welcome.
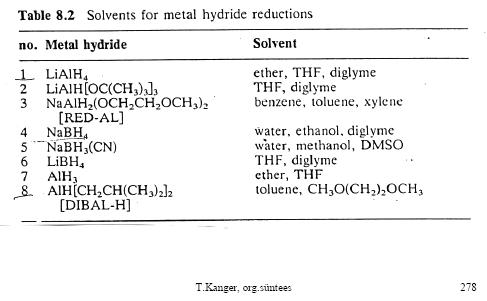
What catalyst should I use to reduce the tri-ester?
And I have some tables here. Thought they might be useful for others aswell.
[Edited on 3-7-2007 by DDoS]
[Edited on 3-7-2007 by DDoS]
Heaven is a half-pipe
|
|
|
DDoS
Harmless

Posts: 11
Registered: 13-5-2007
Location: Estonia
Member Is Offline
Mood: enthusiastic
|
|
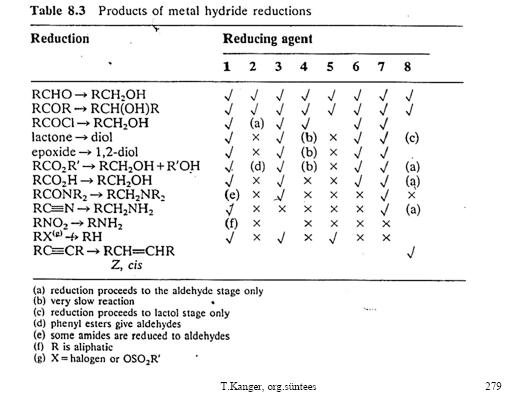
Sorry couldn't put them into 1 post.
Heaven is a half-pipe
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by DDoS
What catalyst should I use to reduce the tri-ester? |
Ruthenium is sometimes used for hydrogenation of carboxylic acids to alcohols. However, the conditions are more than extreme and inaccessible in
laboratory conditions. If you have access to industrial capacities, even some other catalysts like copper chromite are supposed to work (at about
150°C and something like 200 bar!). The same problem is valid for the esters.
What is this about aldol condensations? Your type of compound certainly does not look like being easy to prepare trough aldol condensations. You could
at most make 1,5-dihydroxypentan-3-one (1,3-dimethylol-acetone) from acetone and formaldehyde (see the patent literature), but there is not much
choice on any hydroxymethyl synthons to add at the carbonyl group. Though it is possible, for example through methylenation of the carbonyl with a
Wittig reaction and then epoxidation, it would require laborious conditions and protecting group chemistry… nothing cheap and simple
only things that make the citric acid and LiAlH4 or triethyl citrate and diborane look really good.
Beware that polyols like that can be a bitch to isolate due to their tendency to preferentially partition in the water phase.
[Edited on 4/7/2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|