| Pages:
1
2 |
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
NEW!! Dakin-West Synthesis of B-Aryl Ketones
I know that many here will not appreciate my sharing my latest find... However, this board exists for the purpose of sharing the details of such
serendipitous discoveries - thus - BITE ME
Is it possible to get hold of the full text of this article? The supporting information accompanying which rather jumped out and grabbed me, it appears that by using Methyl Imidazole the authors have achieved the previously unimaginable - getting 94% yield from the decarboxylative acylation of phenylactic acid
(with acetic anhydride) with minimal side-products...
tup
PS Is this, you beaut, pyridine replacement-catalyst related to creatinine (which can be made)? If so, could it be reduced - even to the extent that Li/NH4OH was used to do so?
[Edited on 2-6-2007 by tupence_hapeny]
We are all the sum of our experiences, and our reactions to the same
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Dakin-West Synthesis of B-Aryl Ketones
Khanh-Van Tran and David Bickar
J. Org. Chem., 71 (17), 6640 -6643, 2006.
PS: We have a section for requesting articles.
Attachment: Dakin-West Synthesis of â-Aryl Ketones.pdf (100kB)
This file has been downloaded 2691 times
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I thought that the claims of a new process had been mentioned before.......
| Quote: | MEXCHEM2006
Harmless
*
Posts: 32
Registered: 22-8-2006
Location: MEXICO
Member Is Offline
Mood: ORGANIC SYNTHESIS AND BIOTECNOLOGY
posted on 26-9-2006 at 06:24 PM Reply With Quote Report Post to Administrator
You can mix PAA , acetic anhydride , 1-methylimidazole or bezimidazole under a nitrogen atmosphere and get yields in the range of 93-98%.
|
..............however, the reference was never posted, credit were credit is due...........java
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
In this reaction ..what is the purpose of the nitrogen environment and can Argon be used in it's place?.....also since the methylimidazole is used as
a catalyst how can one recover it and reuse it?.......this must be a very interesting synthesis as 213 members acquired the study, so there must be
someone with some knowledge of the reaction.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
very interesting.
can other anhydrides be used in this reaction, like propionic and butyric anhydride?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I think the methylimidazole act exactly like DiMethylAminoPyridine (DMAP) during a Steglich esterification, so it could possibly be substitued by DMAP or equivalent.
I guess other anhydride could be used, butthe formation of the mixed anhydride might be more difficult, needing longer reaction times and possibly
decreasing yields.. but it's just speculation.
Here is my interpretation of the mecanism; I'd appreaciate if someone more knowledgeable on the subject would give his advice on it:
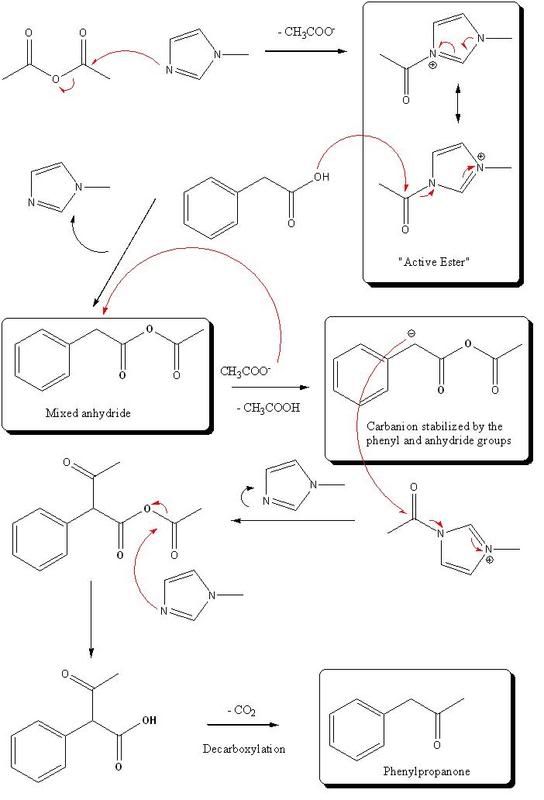
First there is formation of a reactive amide "Active ester" by reaction of acetic anhydride and Methyl imidazole. This species, which is more reactive
than the anhydride itself, forms a mixed anhydride with phenylacetic acide. This mixed anhydride posses very acidic benzylic protons, as the carbanion
formed would be stabilized by the phenyl group and the anhydride, so a base such as the imidazole or acetate can abstarct a proton easily. The
carbanion formed is then acylated by the reactive amide, and the compound formed undergoes decarboxylation after acylating some more Methylimidzole,
to yield the phenylpropanone.
Possibly the use of acetic anhydride could be substitued by using N,N'-Dicyclohexylcarbodimide (DCC) which forms a reactive species similar to an anhydride with an acid: see the Steglich mecanism. But again that's pur speculation on my part

\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
I believe that would pretty much be the right mechanism. Here 1-methylimidazole acts as a nucleophilic catalyst. Pyridine and 4-dimethylaminopyridine
are the usual nucleophilic catalysts used in acetylations etc, forming the highly reactive acetylpyridinium cation in those cases. The "active ester"
would be called 1-methyl-4-acetylimidazolium ion.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Those who are slavering for methyl benzyl ketone (P2P) might find this route a bit arduous. Their time tested exploitation of phenylacetic acid (by
dry distillation with lead acetate) gives that product directly while this much more elegant method requires acetic anhydride, often difficult to come
by. Also methylimidazole, not so commonplace. I would think that those who have PAA and Ac2O might prefer to stick with pyridine, as opposed to either
the highly toxic DMAP, or the methylimidazole.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
Possibly the use of acetic anhydride could be substitued by using N,N'-Dicyclohexylcarbodimide (DCC) which forms a reactive species similar to an anhydride with an acid: see the Steglich mecanism. But again that's pur speculation on my part
 |
Unfortunately O-acyl-1,3-dicyclohexylisoureas (the RCOOH+DCC products) rapidly rearrange to N-acyl-1,3-dicyclohexylureas upon heating (this, among
other things, is also one of the reasons HOBt is often added - to accept the acyl forming RCOOBt before the rearrangement occurs). The
N-acyl-1,3-dicyclohexylureas are not "active" anymore, but even DCC activated acids are not particularly reactive. I can only think of
carbonyldiimidazole (CDI) as being of possible such use (N-acylimidazoles are about as electrophilic as acid anhydrides). In such case the Dakin-West
reaction might not even require any additional base since imidazole would play its role (N-acylimidazoles in acidic media can get protonated and
became about as reactive as the above discussed N-acyl-N'-methylimidazolonium ions).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
DCC is, in addition, a long notorious skin sensitizer, just ask any peptide chemist and DCC will be on his list of least favorite things to work with
or around.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
I would think methyl lithium would be the way to be honest.
lead acetate though very simple is just not a very nice way to go.
a bit off topic and my personal opinion but if the dark lord can add his
I think I should too.
lead acetate is for those who can not make the PAA in the first place.
other than the obviose aquasition problems this seems to be a nice
way to go.
does any one have any ideas on how to get the methyl lithium reaction
with carboxylic acids to yeild higher than the 70 ish % that is normaly
quoted.
oh and PAA is not just for P2P. truly P2P is a boring substance.
thanx for this thread tupence_hapeny yes we are a closed mouth
lot thats for sure.
but after sassafrass and ephedrine can you blame us 
[Edited on 14-6-2008 by Ephoton]
Edit by Nicodem: Stick to the topic and no more P2P-ing. Deranging this thread further into cookery discourse will make me use the edit
function to remove text instead of adding it.
[Edited on 14/6/2008 by Nicodem]
e3500 console login: root
bash-2.05#
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You are going to hurt Organikum's feelings, I think he likes that hoary old Japanese lead acetate/PAA reaction.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
hehe sorry orgy ;P
peace love and broad beans 
e3500 console login: root
bash-2.05#
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
I think the methylimidazole act exactly like DiMethylAminoPyridine (DMAP) during a Steglich esterification, so it could possibly be substitued by DMAP or equivalent.
I guess other anhydride could be used, butthe formation of the mixed anhydride might be more difficult, needing longer reaction times and possibly
decreasing yields.. but it's just speculation.
Here is my interpretation of the mecanism; I'd appreaciate if someone more knowledgeable on the subject would give his advice on it: ............
|
I do not like this mechanism  . Firstly, PAA reacts with acetic anhydride,
creating mixed anhydride; secondly, it is benzylic (α) position attacked and H is "removed"... etc. It is proven , in this and similar
cases, there must be at least two hydrogen atoms attached to this α position. Besides, it is doubtful at all, that acetate anions acts as
"H-abstractors" and ketene mechanism is suggested. . Firstly, PAA reacts with acetic anhydride,
creating mixed anhydride; secondly, it is benzylic (α) position attacked and H is "removed"... etc. It is proven , in this and similar
cases, there must be at least two hydrogen atoms attached to this α position. Besides, it is doubtful at all, that acetate anions acts as
"H-abstractors" and ketene mechanism is suggested.
Long time ago I found site about "wonderful" DMAP.
http://www.dmapcatalyst.com/
My fellow used to check (at univ. laboratory) this catalyst in reaction MeOH and Ac<sub>2</sub>O : in a moment products of reaction were
outside flask 
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
Hang on, there are suggestions here that other substited n-containing bases would work, so why not caffeine? It has the 1-methylimidazole structure
with one nitrogen free so as to make the n-acyl-imidazole, so why couldn't that be substituted? Be nice if it could, it would be hard to find
something more OTC.
Alternatively, is there someone here who can think of a way to imidazole from histamine/histidine?
Whhhoooppps, that sure didn't work
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for the comments KMnO4 
So you think the acid would directly react with the anhydride in the first place? Sowhne does the imidazole come in place, when the benzylic proton
is abstracted? It's true the amine would surely take the proton long before the acetate would, I worked by analogy from the Perkin reaction etc
Oha nd thanks for the site! I persoanlly have never used DMAP at work, a few colleagues described it as been routinely used in acylations. IIRC Sauron
also mentionne dit was pretty toxic (i like the photo of the beautifull free-flowing DMAP  ) )
Is your comment related to the contents "leaving" the flask in any way related to this:
| Quote: |
Dakin-West Scale-up: A pyrrolopyrimidine was required in 100 Kg quantities for pharmaceutical development. This required preparation of an
acetylaminobutanone intermediate which could be obtained easily in the laboratory by trea®ent of alanine with acetic anhydride and pyridine – the
Dakin-West procedure.4 However, this procedure could not be used safely on a technical scale because of the sudden evolution of a stoichiometric
amount of carbon dioxide in the decarboxylation step. The chemists at Novartis AG developed a procedure that utilized DMAP and TEA5 with
additional acetic acid to provide water to promote azlactone hydrolysis and facilitate decarboxylation. Alanine was added as the limiting reagent to
control carbon dioxide evolution. The result was formation of acetylaminobutanone safely (50 Kg in a 400 L reactor) in greater than 90% yield, with
great reduction in the amount of acetic anhydride used.
|
Could you please direct me to a description of the ketene mechanism? I haven't found any in a quick search.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
I've tried out this very method, using 1-Methylimidazole (MIM) as catalyst. It works great! Just let it stir with at rt and you're set. But of course,
there is a pitfall - as MIM is such a great catalyst, it C-acylates anything that it can when heat is applied - this means that the funky ketone you
just made turns into 1,3-Diphenylacetone. Also, a lot of the enol ester is formed (not much of a problem).
So, the experienced reader may ask himself, how do i get rid of the excess anhydride floating around my mixture?
I haven't yet found a convenient workup procedure that avoids the darkening of the mixture. So far i've tried rotavapping off the acetic anhydride (no
good), vac distillation (quite ok but serious darkening and only moderate yield). I've also done a run with butyric anhydride which (by TLC) yielded
the desired ketone, but the anhydride is obviously a lot more difficult to remove.
Please, dear chemistry lovers, throw in your thoughts!
btw: As it turns out, MIM will also O- and N-acylate anything. So don't tell the chinese heroin cooks!!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by stoichiometric_steve
So, the experienced reader may ask himself, how do i get rid of the excess anhydride floating around my mixture? |
Like quenching with dilute aq. ammonia solution?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Nicodem, i should have mentioned - i want to save excess anhydride, so i guessed that an aqueous workup would usually involve the inavoidable
hydrolysis of Ac2O. Am i correct?
[Edited on 8-1-2009 by stoichiometric_steve]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I have herd from some friends that the dakin west reaction using methylimidazole works fine ....with an 18 hour reflux and at the end cool it in an
ice bath and deactivate the imidazole with molar amount of conc. Hcl......then water can be added and Naoh to liberate the ketone.....the reaction was
run without a nitrogen environment the yields were in the 80%.......unfortunately the acetic anhydride cannot be recovered ......but the ionic
imidazole Cl can be recovered and used in a chlorination project......my few cents on the subject.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by solo
I have herd from some friends that the dakin west reaction using methylimidazole works fine ....with an 18 hour reflux |
sorry solo, i followed my reactions by TLC and there was absolutely no need for reflux. the starting material was gone after 24h at ambient temp!
refluxing the mixture will only generate 1,3-diphenylacetone and severe darkening of the mixture. if MIM was deactivated by HCl, how do you think it
would be able to act catalytically if there is such a lot of AcOH around? it is a strong base, after all...
i am led to believe that your "friends" have either not performed the reaction or did not characterize their products. i did.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: |
i am led to believe that your "friends" have either not performed the reaction or did not characterize their products. i did. |
So far I cannot see any characterisation of products or even your procedure (cat/anh/acid ratios).
I cannot also understand one thing - how "funky ketone" can be acylated to dibenzylketone ?
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by kmno4
| Quote: |
i am led to believe that your "friends" have either not performed the reaction or did not characterize their products. i did. |
So far I cannot see any characterisation of products or even your procedure (cat/anh/acid ratios).
I cannot also understand one thing - how "funky ketone" can be acylated to dibenzylketone ? |
"funky ketone" benzylic hydrogen is a lot more acidic than acetic anhydride alpha hydrogen. check the mechanism in the paper!
oh yeah, other than that, i tried the method laid out in the paper and characterized the product by TLC comparison with an authentic sample, as
already mentioned above. if only some people cared to read
[Edited on 9-1-2009 by stoichiometric_steve]
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: |
"funky ketone" benzylic hydrogen is a lot more acidic than acetic anhydride alpha hydrogen. check the mechanism in the paper!
|

If it really belongs to acidity of H atom bonded to C - all "funky ketone" will turn into its acylated derivatives, no matter if it is refluxed or
not. Besides, acylation of this ketone (at benzylic position) would give only phenyl derivatives of acetylacetone.
Characterization means (at least for me) also % amounts of output products. It also is good to know something about inputs.
Few cents of Solo tell more (18 h reflux/yields 80%) than yours.
Great method ! - without numbers it means nothing.
I know a few great methods giving 90% yields, but only on paper.....
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by solo
I have herd from some friends that the dakin west reaction using methylimidazole works fine ....with an 18 hour reflux and at the end cool it in an
ice bath and deactivate the imidazole with molar amount of conc. Hcl......then water can be added and Naoh to liberate the ketone.....the reaction was
run without a nitrogen environment the yields were in the 80%.......unfortunately the acetic anhydride cannot be recovered ......but the ionic
imidazole Cl can be recovered and used in a chlorination project......my few cents on the subject.......solo |
.........after sharing the comments on this thread to my friends , it turns out that `i was wrong in stating the reaction was refluxed with
heat......as it was done at RT as steve mentioned and is correct, but the remaining information about adding water to kill the reaction then adding
and hcl to separate the base was on track along with the yields,.....sorry about my confusion, but at last it's been corrected.......solo
[Edited on 12-1-2009 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
| Pages:
1
2 |