Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
Does anyone have solubility data of HCl in various solvents (Methanol, Ethanol, Ether)?
I've been searching for the past 20 minutes and to my surprise I can't find any data about this. At best I can find companies selling premixed
solutions of different concentrations, but this doesn't tell me much about solubility itself. I am mostly interested in it's solubility in Methanol.
Any information is appreciated.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Why doesn't it tell you much about the solubility itself? Aldrich sells a 3M solution of HCl in methanol. Are you trying to make the most
concentrated solution possible, is that your question? Because otherwise 3M is a good data point.
They also have a 2M in ether and a 1.25M in EtOH which are again good data points to have.
A quick search though gives the following information:
| Quote: | Solubility in methanol: 54.6 g/100 g solution at -10 deg C; 51.3 g/100 g solution at 0 deg C; 47.0 g/100 g solution at 20 deg C; 43.0 g/100 g solution
at 30 deg C; solubility in ethanol: 45.4 g/100 g solution at 0 deg C; 42.7 g/100 g solution at 10 deg C; 41.0 g/100 g solution at 20 deg C; 38.1 g/100
g solution at 30 deg C; solubility in ether: 37.52 g/100 g solution at -10 deg C; 35.6 g/100 g solution at 0 deg C; 24.9 g/100 g solution at 20 deg C;
19.47 g/100 g solution at 30 deg C
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 888
|
[Edited on 7/16/2018 by BromicAcid]
|
|
|
Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
Pretty much. I still plan to titrate after making it the fact to determine what concentration it ends up at, but it would still help me a lot if I had
a rough idea of how much I can expect the methanol to hold. I prefer to have as much data as I can before I go into things like this to avoid wasting
reagents. Something as common as HCl, had me surprised I wasn't able to more easily find a solubility table/chart online.
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
I found this.
https://srdata.nist.gov/solubility/IUPAC/iupac.aspx
Hopefully, this will help you.
Edit: I could not easily find the data for HCL, but it appears to be here.
https://srdata.nist.gov/solubility/IUPAC/SDS-42/SDS-42.pdf
[Edited on 16-7-2018 by MJ101]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
This is given on p. 50 of "Purification of Laboratory Chemicals" by Armarego and Perrin (6th Ed.):
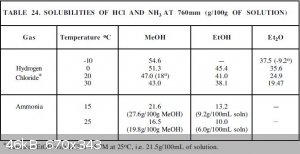
|
|
|
Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
I looked over the data from multiple of the above posts (thank you everyone), and if I am interpreting them correctly, Hydrogen Chloride can dissolve
into Methanol at up to a 45-50% concentration? Isn't it's water solubility capped around 37% or so before it starts fuming? I didn't imagine Methanol
would be able to achieve such a high concentration.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
100g of methanol has a larger volume than 100g of water due to differences in density. Essentially you're dissolving the same mass of gas (HCl) in a
larger volume of solvent, hence the larger %w/w concentration.
If you normalise for density of the solvent then both water and methanol both give HCl solutions around 37%w/v
|
|
|