Ex Nihilo
Harmless

Posts: 3
Registered: 8-11-2016
Member Is Offline
Mood: No Mood
|
|
Starting from benzene...
Good day!
Can you help me understand the attached synthesis circuit?
Will all the described reactions proceed, which way to go is better and why?
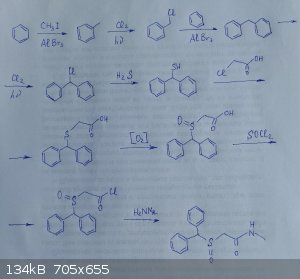
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Is this a school assignment or a synthetic scheme you wish to carry out? Cause I don't see why you wouldn't just start from toluene to skip the first
step, which, if I'm not mistaken, is hard to limit to only a mono-alkylation since alkyl groups are activating in EAS.
There are some other steps I don't believe at the moment but they're certainly interesting reactions and I may have just never seen them before, it
could just be my inexperience *shrug*
EDIT: Also the third transformation I'm fairly sure won't work. Are you thinking of it as a friedel-crafts alkylation? Cause I was taught those won't
proceed with vinyl or *aryl* halides since their cations are too stable.
[Edited on 27-6-2018 by JnPS]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
1.like JnPS said,in the first step there will be overalkylation and the yield of toluene will be very bad (1.5%)-http://www.umich.edu/~chemh215/CHEM216/HonorsCup/HC%20220-II...
2.You can make thiols from H2S -https://pubs.acs.org/doi/pdf/10.1021/jo00403a014,but the safer and less stinkier way is to use thiourea.
3.For showing oxidation,you write [O] instead of [O2],unless you want to do the oxidation with O2-https://www.organic-chemistry.org/abstracts/lit4/114.shtm
overall the synthesis is correct theoretically, but practically its wrong since its too long and your final yield would be in single digits.The same
product can be made in 2 steps starting from benzhydrol,or in 1 step from modafinil 
are you trying to do something similar to nurdrage's daraprim synthesis,by making a drug from scratch using OTC reagents ?Because there are better OTC
starting compounds than benzene
Quote: Originally posted by JnPS  | | Cause I was taught those won't proceed with vinyl or *aryl* halides since their cations are too stable. |
You
mean their cations are too "unstable".But this is a benzyl halide.
[Edited on 27-6-2018 by CuReUS]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
It would be a lot easier to make Benzyl Chloride starting with Benzyl Alcohol and HCl.
The Grignard reaction of BromoBenzene with Magnesium, and then with Benzaldehyde, might be better still. Produces an alcohol. Reaction of the
resulting Alcohol with HCl, takes you pretty far along.
Then perhaps, Sodium Hydrogen Sulfide, could be reacted with the Halogen. to produce your Organosulfide.
From a practical standpoint, you must optimize yields, and minimize reaction steps. Otherwise, a tractor trailer load of reagents, might produce only
a thimble full of product. After six steps, at so-so yield, there ain't much beef left.
And, in my rough estimation, there are actually more than 6 steps involved.
50%x50%x50%x50%x50%x50%= .5 to the 6th power=.015625=1.5625% over-all yield.
Now, it's pretty noisy around me right now, and I may be miscalculating, but the principle is still present. Minimize reaction steps, and Maximize
yields.
--------------------------------------------------------------------------------
OK, I checked the WEB. Some kind of prescription alertness enhancer.
I don't always catch on, right away. But, depending upon your location, synthesis may be proscribed by local laws.
As luck would have it, its synthesis was discussed at length, back in the Hive/Rhodium era. Reagents that I wasn't sure existed, do exist, and
reasonable synthetic pathways have been well mapped out.
https://erowid.org/archive/rhodium/chemistry/adrafinil.modaf...
[Edited on 28-6-2018 by zed]
[Edited on 28-6-2018 by zed]
[Edited on 28-6-2018 by zed]
[Edited on 28-6-2018 by zed]
[Edited on 28-6-2018 by zed]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Ex Nihilo  | Good day!
Can you help me understand the attached synthesis circuit?
Will all the described reactions proceed, which way to go is better and why?
|
Where did you get that? Is it for a class? No amateur chemistry is performed "starting from
benzene", as purchases of benzene are restricted to professionals because of its carcinogenicity.
If you wrote it yourself, my advice is: you're not ready to attempt the synthesis of modafinil.
However, for the purposes of our amusement, I'm pretty sure that the "just stir it" method of making acetamide from ethyl acetate could also be
applied to make thioglycolamide from ethyl thioglycolate. If you can esterify thioglycolic acid with ethanol, stirring this ester with aqueous ammonia
should produce thioglycolamide. This then reacts with benzhydryl chloride or bromide to give the desired compound, short an oxygen atom. This 2-step
procedure should be much easier (and safer) than trying to produce thioglycolamide by heating ammonium thioglycolate, as thiol-containing carboxylates
sometimes decompose to release H2S
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
And that's not even modafinil that's drawn. OP drew the N-methyl derivative.
Benzhydrol would be a much better starting point than benzene.
[Edited on 6-28-2018 by Metacelsus]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The first few steps could be replaced by the FC rxn of benzene and dichloromethane.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Ex Nihilo
Harmless

Posts: 3
Registered: 8-11-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
are you trying to do something similar to nurdrage's daraprim synthesis,by making a drug from scratch using OTC reagents ?Because there are better OTC
starting compounds than benzene |
Actually, yes! I want to attempt the synthesis of N-methyl-modafinil from the scratch similar to nurdrage's daraprim synthesis in educational purpose.
I really like to think about different syntheses from the scratch - starting from very basic components or even OTC reagents. So my goal is not a
viable amount of the end product but to do all the steps in the lab, starting from this theoretical issue I made on my own mention and ending with
positive NMR results which will confirm a success of the overall process.
One of the most important deeds in my wish-list - to synthesise the toluene from benzene using F-C alkylation.
The reason why I use CH3I instead of CH3Cl - because of its easiest handling (liquid instead of gas) and as I think more easiest synthesis. The AlBr3
seems for me more simple in making instead AlCl3 or even other Lewis acids (if anyone knows a better reagent just give me know).
So, if you have any idea on how to make toluene from the scratch I would appreciate your help. Maybe it will not be in one step, maybe it took more
than two or three but if get overall yield about 10% or higher it will be ok!
I was thought about that.
Starting from methyl ketone ( R(C=O)CH3 , R= CH3, C2H5, etc ) via Haloform reaction making chloroform and then reducing chloroform with hydrochloric
acid and zinc metal in ethanol to obtain dichloromethane.
But I did not have any synthesis references on that so if anyone has one - I will be grateful!
Quote: Originally posted by clearly_not_atara  |
However, for the purposes of our amusement, I'm pretty sure that the "just stir it" method of making acetamide from ethyl acetate could also be
applied to make thioglycolamide from ethyl thioglycolate. If you can esterify thioglycolic acid with ethanol, stirring this ester with aqueous ammonia
should produce thioglycolamide. This then reacts with benzhydryl chloride or bromide to give the desired compound, short an oxygen atom. This 2-step
procedure should be much easier (and safer) than trying to produce thioglycolamide by heating ammonium thioglycolate, as thiol-containing carboxylates
sometimes decompose to release H2S |
Thank you very much!
I will study this information in some detail.
[Edited on 28-6-2018 by Ex Nihilo]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
or with formate ester to give benzhydrol directly 
| Quote: | | Then perhaps, Sodium Hydrogen Sulfide, could be reacted with the Halogen. to produce your Organosulfide. |
hydrosulfides tend to give disulfides rather than thiols.
Can you do it in one step using TCT ?-https://www.sciencedirect.com/science/article/pii/S004040390... Quote: Originally posted by Ex Nihilo  |
I was thought about that.
Starting from methyl ketone ( R(C=O)CH3 , R= CH3, C2H5, etc ) via Haloform reaction making chloroform and then reducing chloroform with hydrochloric
acid and zinc metal in ethanol to obtain dichloromethane.
|
DCM is OTC 
your craze for trying to synthesize compounds "ex nihilo" is really getting to me 
[Edited on 29-6-2018 by CuReUS]
|
|
|