| Pages:
1
2 |
RogueRose
International Hazard
    
Posts: 1594
Registered: 16-6-2014
Member Is Offline
|
|
Retort vs distiling flask (vertical neck w/y downward angled side arm) - differences?
So I am wondering if there is much difference between these two items. I was thinking of making an adapter that can fit onto a flask with 1-3 necks
and then a vertical shaft that has a narrow downward arm coming off of it, like in the picture of the distilling flask, except that it would be 2
pieces.
 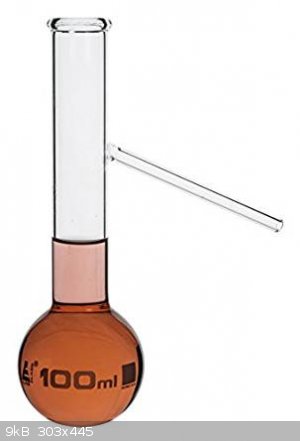
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
There are still heads/thermometer adapters that do the same thing, with three joints (one for a condenser or bubbler tube), but I haven’t come
across one with just a narrow tube. But anyways, there isn’t really much different, the one on the right is just the modern version of a retort IMO.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
RR, I assume you know that neither set-up will distill more than a few cc. without connecting to a condenser...
|
|
|
Texium
Administrator
       
Posts: 4583
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Both are primative, inferior pieces of glassware compared to modern standard taper equipment.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
The item on the left is a reaction flask and integral air condenser. Typically in the old days used for distilling high temperature boiling or
corrosive liquids such as sulphuric acid, nitric acid and mercury because it’s all glass.
The item on the right is a reaction flask with integral take off side arm. Typically in the old days a bung with a thermometer would be inserted in to
the top to monitor the temperature of the distillate or the contents of the flask depending on the position of the thermometer bulb. The side arm
would be attached to a condenser with an other bung.
I am gobsmacked you have not seen pics similar to the ones below in old books.
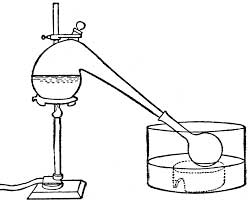 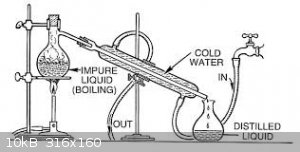
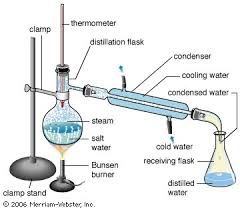
[Edited on 15-6-2018 by wg48]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I used to own a decent sized side armed distilling flask. Perhaps a 2000-3000 ml unit.
In days of yore, such items were dirt cheap. Not so nowadays. You can still buy 'em, but they are spendy. To my recollection, within the last year
or so, one of our members obtained one constructed of Quartz..... For the purpose of distilling Sulfuric Acid, etc..
On occasion, I heated mine with a Bunson Burner, and thereby steam-distilled benzene/water out of a reaction mix.
I don't know that I would have attempted that, with regular jointed glassware.
Probably not.
Well sealed, it seemed pretty safe for distilling flammable solvents. Though of course, not ether.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
For bumpy distillations or high temp ones at reduced pressure where the impurities are of close BP to your target product you can put a fractional
column in there too. That way if it does bump then it goes up and down the column and not over to your pure product. You can get more control of it
with a column in there too imo.
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If you've got two of them, you put the thin output tube of the one being heated through a cork in the top of the other one (all the way down to the
bulb)and cool it with an air blast, or even water poured over the bulb.
This is very old school, but works if the Boiling point isn't too low.
(Be sure to trim your corks and treat them with collodion if you're trying a vacuum distillation this way, and you might want to grow a handlebar
moustache to complete the whole 1890s chemist image)
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
Are we sharing terrible improvised distillation equipment now? My favorite was always the pyrex bowl inside a stew pot with its lid upside down with
ice on top. Put your thing to be distilled into the stew pot, and let it condense into the bowl. Everyone's got distillation apparatus at home!
This is spectacularly quick and dirty, but does work in practice. Not that I ever want to work that way again...
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Nothing improvised about the double flask thing.
It was a common old practice, and illustrations can be found of this setup pretty easily.
If you're curious, try Fieser and Fieser's lab manual, Organic Experiments.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yup. If I had a couple of 'em now, I'm sure I would find uses for them.
But, as stated before, those sidearm distilling flasks, are no longer easy to locate, and even more troubling..... They are kind of expensive.
Though.... I do know a friendly glassblower. Hmmmm.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by SWIM  |
If you've got two of them, you put the thin output tube of the one being heated through a cork in the top of the other one (all the way down to the
bulb)and cool it with an air blast, or even water poured over the bulb.
|
An air blast is a piss-poor alternative to circulating water and cooling the receiver with water needs water constantly running onto it from
the tap...
|
|
|
coppercone
Hazard to Others
  
Posts: 133
Registered: 5-5-2018
Member Is Offline
|
|
Nah these aee not inferior their just specialized. If you want to torture glassware say for distillation of sulfuric acid you cant beat an air cooled
retort that is one piece of glass.. Nothing esle stands up well to high temp distillation because you get seized joints and shit
For h2so4 distillation i would like a pear shaped retort i can do direct flame heating on for the first run. I got super paranoid about glassware
cracking when i did it, i used a hempel column as a condesner and a j joint but everything seized up with vacuum grease and i was banging on it with a
rubber hammer and a gas torch to seperate it.
I think i put a fan to cool my air condesner but with a boiling point of 337c you probobly want to use a air heater to cool it to maintain some kind
of reasonable gradient of temperature. I was paranoid about glass cracking. It should be vacuum distilled but purifying a gallon of drain cleaner is
industrial chemistry imo at least for the first pass. I like using a blowtorch with the air holes mostly clogged to make a gentle flame that waves
around the glass
[Edited on 21-6-2018 by coppercone]
[Edited on 21-6-2018 by coppercone]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Air blasts work fine for what they are appropriate for.
Water, however, is a piss poor cooling medium for anything where the water would make for excessive thermal stress on the condenser.
As for cooling the receiver with water, yes exactly like the diagrams I mentioned above.
Glad you were able to interpret that diagram properly, but I would have assumed you were able to do so anyway.
After all, it is just a basic lab manual...
And just out of curiosity, have you figured out yet that your condenser requires water as well?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by coppercone  |
For h2so4 distillation i would like a pear shaped retort i can do direct flame heating on for the first run. I got super paranoid about glassware
cracking when i did it, i used a hempel column as a condesner and a j joint but everything seized up with vacuum grease and i was banging on it with a
rubber hammer and a gas torch to seperate it.
|
The problem with distilling H2SO4 is that if the flask cools a tad too quickly from 339° the stresses introduced will likely
cause it to fail during subsequent runs ─ If I were to distill sulphuric acid I'd want some way of annealing the glass and to prevent
joints seizing I'd lubricate with the conc. acid...
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by SWIM  |
And just out of curiosity, have you figured out yet that your condenser requires water as well?
|
Just out of curiosity; are you mentally retarded or what ─ if that's not a leading question?
|
|
|
coppercone
Hazard to Others
  
Posts: 133
Registered: 5-5-2018
Member Is Offline
|
|
Quote: Originally posted by hissingnoise  | Quote: Originally posted by coppercone  |
For h2so4 distillation i would like a pear shaped retort i can do direct flame heating on for the first run. I got super paranoid about glassware
cracking when i did it, i used a hempel column as a condesner and a j joint but everything seized up with vacuum grease and i was banging on it with a
rubber hammer and a gas torch to seperate it.
|
The problem with distilling H2SO4 is that if the flask cools a tad too quickly from 339° the stresses introduced will likely
cause it to fail during subsequent runs ─ If I were to distill sulphuric acid I'd want some way of annealing the glass and to prevent
joints seizing I'd lubricate with the conc. acid...
|
yes which is why I said you should aim a heat blower at it for cooling if you want to repeat my work with less danger
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Of course you should use an air-condenser for distilling HB fluids, but in this case, blasting it with air is neither necessary nor desirable...
The only 'danger' is a RBF that's been compromised, obviously...
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
My, how very, very sad.
Throwing a hissy-fit is a poor way to react when you can't understand the question.
I realize puberty can be a trying time, and these new feelings you're having can be very confusing, but taking your frustrations out on the world
around you is not only inappropriate, but a rather tired cliche as well.
You may appreciate this further when you mature somewhat.
|
|
|
Texium
Administrator
       
Posts: 4583
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Please
refrain from ad hominem. We're better than this.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by SWIM  |
I realize puberty can be a trying time, and these new feelings you're having can be very confusing, but taking your frustrations out on the world
around you is not only inappropriate, but a rather tired cliche as well.
|
Oh dearie me! Little closet-boy's got his panties twisted knotwise...
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
You know me, Texium ─ I can tolerate most anything except wilful ignorance...
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think you could do short path distillation at temperatures below the boiling point of the mixture with a retort. Distillation flasks have more
options than retorts, but generally speaking, you probably don't want to buy either for chemistry use unless you can get an exceptionally good deal or
have a specific application. (For example, I have a standard taper quartz distillation flask for distilling sulfuric acid.)
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
I use both retorts and distillation flasks. The former I use mostly for nitric acid, and also as the first stage condenser for sulfuric acid
(injecting hot, not precooled H2SO4 vapor into a water-cooled condenser = bye bye condenser).
Smells like ammonia....
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by SWIM  | My, how very, very sad.
Throwing a hissy-fit is a poor way to react when you can't understand the question.
I realize puberty can be a trying time, and these new feelings you're having can be very confusing, but taking your frustrations out on the world
around you is not only inappropriate, but a rather tired cliche as well.
You may appreciate this further when you mature somewhat.
|
[moderator hat is on]
SWIM and hissingnoise - cut it out!
We just don't need this. Nor do we need you justifying yourself, hissingnoise.
[/mod hat]
|
|
|
| Pages:
1
2 |