DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Molecular Sieves
Okay- I really don't know much about molecular sieves. They're great for drying stuff, but that's about all I can tell you.
Now, I'm looking at making some esters, and I don't have a Dean-Stark trap (and they're expensive). It occurred to me that it might work to take a
Vigreaux column (or similar bit of glassware), pack it with molecular sieves, and allow them to suck the water out of the system during the reflux.
Anyone who knows more about such sieves care to opine on this?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
walruslover69
Hazard to Others
  
Posts: 233
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
Sieves are slightly basic and will be attacked by acid so it probably isn't compatible for an acid catalyzed esterification.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Yes, but they'll be in the column, where they'll be in contact with the alcohol and water vapours, not the non-volatile catalyst acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The Dean-Starks I have seen, seem fragile and expensive. Also, they depend on using a solvent immiscible with the water being removed. The Soxhlet
can generally do the same thing, but it has further applications.
https://www.youtube.com/watch?v=Ah5ds_3s5BI
500 ml, is actually a pretty big unit.
https://www.ebay.com/itm/500ml-24-40-Glass-Soxhlet-Extractor...
Might be too big for the scale of experiment you are planning.
Smaller units are less expensive.
[Edited on 15-5-2018 by zed]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
This has been done before... If you would use sulfuric acid or so as catalyst it should work fine.
https://www.google.nl/search?rlz=1C1EJFA_enNL769NL769&q=...
|
|
|
walruslover69
Hazard to Others
  
Posts: 233
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
It would probably work the way you described but isn't optimal.
1) drying by Just packing a reflux column with sieves is going to be very slow. In a soxhlet you are cooling and condensing the vapor. Sieves work
slower at higher temperatures so they will absorb water slower in a reflux column. In a soxhlet the sieves are allowed to sit in the condensed liquid
vs having the condensation constantly dripping down in your reflux column, slowing adsorption even further.
2) You still might have problems with your carboxylic acid dissolving the sieves. Carboxylic acids smaller than pentanoic acid have a azeotrope with
water with a boiling point of around 100C, this is higher than the water/alcohol azeotrope but only by 10-20 degress so without a fractional coloum
the vapor and condensate you are passing over your sieves contains acid. In a soxhlet the condensate is cooled to room temperature acid reacts very
slowly, but with a reflux column your sieves will be in constant contact with a dilute solution of boiling acid. I doubt it would harm your reaction
but its a waste of good sieves.
3) would it not just be more efficient to just ad an excess of cheap sulfuric acid? anhydrous copper sulfate would also make a faster and superior
drying agents to pack a reflux column with.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The trouble with using other drying agents such as copper(II) sulphate or calcium chloride is that the column wouldn't stay packed with it- they'd
dissolve at least somewhat in the partially aqueous mix and fall into the reaction mixture.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
walruslover69
Hazard to Others
  
Posts: 233
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
That hadn't occurred to me. Would that really be a problem though? I imagine you are doing to distill your product anyways. Thinking about it now, I
don't see a problem with just adding your drying agent directly. The only reason sieves aren't added directly is because of the acid.
What kind of esters are you looking into making? just out of curiosity.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
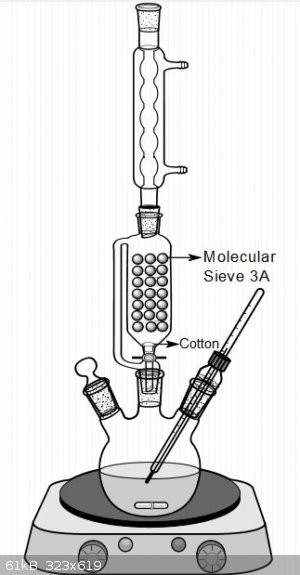
I usually use this system for making ethyl ester of different carboxylic acids.Also this is possible to use it for another alcohol if you can make
azeotrope for bring out of formed water.
[Edited on 17-5-2018 by Waffles SS]
[Edited on 17-5-2018 by Waffles SS]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Yeah, I'd go with the addition funnel if I had one, but right now I'm trying to make do. And addign the drying agent right to the reaction mixture
won't work as well, because it will be at a temperature where it's not absorbing water very well. I'm mostly looking at benzoates and salicylates,
which have too high bp to effectively distill.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
What ester do you want to make?
Maybe there is better and cheaper method for synthesis it
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
MAKE ALL THE ESTERS!!!
That basically sums it up......
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Usually adding excess sulfuric acid as Esterification catalyst and water absorber work fine for synthesis most of esters. I made many esters by this
mothod.
Below link is synthesis of Dimethyl oxalate by this method.
http://www.orgsyn.org/demo.aspx?prep=CV2P0414
But if you are still interest to use molecular sieve then i think there is certain type of it that is resistance to acids.just search it
[Edited on 18-5-2018 by Waffles SS]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Yeah, but the more esters I try, the more I find that things that should work fine don't really seem to work for me.....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I have thoughtfully designed and executed a lot of clever experiments, in numerous areas.
At least, I thought they were clever, at the time.
I almost always failed.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
You don't need to buy an actual dean stark trap. I use a few adapters that I already had to achieve the same goal (and more flexibly than if i'd
bought a real dean-stark trap). Basically, a bent adapter from the boiling flask, which goes to the side arm of a still head/3-way adapter. Reflux
condenser connected to the top of the 3-way adapter and a small flask to the bottom, where the boiling flask would normally go. You can also hook up a
small sep funnel, a hose connection w/ stopcock, etc.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If you pick something simple, like wintergreen, i'll have a go at replicating your setup and see what happens.
Hardly any -ols here tho.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by DavidJR  | | You don't need to buy an actual dean stark trap. I use a few adapters that I already had to achieve the same goal (and more flexibly than if i'd
bought a real dean-stark trap). Basically, a bent adapter from the boiling flask, which goes to the side arm of a still head/3-way adapter. Reflux
condenser connected to the top of the 3-way adapter and a small flask to the bottom, where the boiling flask would normally go. You can also hook up a
small sep funnel, a hose connection w/ stopcock, etc. |
I have done similar by just using the distillation head on the neck of the flask, with a condenser where the thermometer normally goes, and a flask
where the condenser would normally go.
The flask has to be at an angle so the collection flask is down and the condenser up, but it does work. The distallate runs down the angled condenser
into the collection flask and overflows back to the reaction pot.
|
|
|