vannylaholic
Harmless

Posts: 20
Registered: 3-7-2017
Member Is Offline
Mood: inquisitive
|
|
Amino ketone formation ( looking for differing opinions on ketonizations
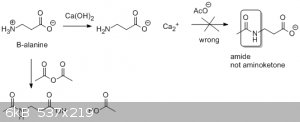
So I plan to take beta alanine and form the calcium salet and react it with the molar equivalent of calcium acetate , I suspect an amino ketone will
form. But I have a feeling that amino group isn't going to fare well under extreme heat needed to distill the calcium salts . any helpful ideas or
flaws in my theory before I go for it
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
What i think the OP is attempting to do (judging by his post history), is the following reaction, based off the pyrolosis of calcium acetate to form
acetone.
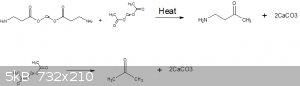
Unfortunately the reaction is unlikely to proceed the way you want and would likely lead to some acetone being your only distillate, which you may be
able to discern by looking at that sketch but i will clarify.
The calcium salt is able to undergo decarboxylation like that with the acetate because the calcium cation is bound up between the two acetate anions
due to calciuims +2 oxidation state.
This of coarse would not occur with sodium or potassium salts with a +1 oxidation state because this decarboxylation is an intramolecular reaction,
not an intermolecular reaction.
Thus its not possible for a mole of calcium acetate and a mole of calcium alanine to condense together.
However, If you were to instead try to pyrolize the following salt then your reaction would theoretically work.

However this still may not work, as the reaction is dependent on the volatility of the final product, as well as how stable it is towards high
temperatures.
I did find the following in regards to 4-amino-2-butanone.
http://www.chemspider.com/Chemical-Structure.14185695.html
which indicates a BP of 155*C, which is well below the operating temperature of the reaction. This may work, but would be dependent on how well that
ketone would be able to handle those extreme temperatures.
An inert atmosphere may also help to prevent any oxidation which would be disastrous.
|
|
|
vannylaholic
Harmless

Posts: 20
Registered: 3-7-2017
Member Is Offline
Mood: inquisitive
|
|
Thanks all I will run the experiment and post my results .
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
mea coulpa, the "Beginnings" section made me misunderstood the question 
|
|
|
vannylaholic
Harmless

Posts: 20
Registered: 3-7-2017
Member Is Offline
Mood: inquisitive
|
|
So in a small scale of 1 gram ,I formed calcium alanine and preformed the pyrollsys to my surprise there was no distillate , but only a faint aroma of
an amine like (very rotten fisn) aroma. I decided that its was more interesting and sensible to just react the calcium alanine instead of the calcium
acetate and alanine. Could have I produced an alkyl amine of some sort , or is this similar to the aroma of of 4amino 2butanone , I can't find an
aroma description anywhere ,
|
|
|
vannylaholic
Harmless

Posts: 20
Registered: 3-7-2017
Member Is Offline
Mood: inquisitive
|
|
Or could the amino ketone cyclize to 1methyl piperidine from the excessive heat,
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I was actually thinking it would instead polymerize and form black tar, as the heat would be enough to drive off water and dehydrate the product.
If it were cyclizing i would instead expect some kinda distillate.
The presence of an ammonia or alkyl amine aroma would more likely indicate decomposition of the product, but this is far from a qualitative way of
determining any kinda reaction.
If the reaction you attempted were successful then i would expect the following product. 1,5-diamino-3-pentanone.
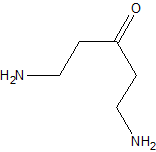
http://www.chemspider.com/Chemical-Structure.11373728.html
According to that reference this compound should have a boiling point of 243*C.
However the temperature for the reaction might be too high for the compound to be stable, what temperature did you manage to get your boiling flask
too?
Also did you see any water condensation at all in your flask?
If you weren't able to roughly determine the temp of your pot then i would suggest trying again and using at the very least one of
those laser pointer thermometer things to get a rough idea of the pot temp.
Most thermocouples can handle temps that high but those laser dot thermometers are relatively accurate and should work fine for those kinda
temperatures.
It might also be a good idea to try this in a steel distillation set up, if i recall watching NileRed's video when he made acetone, where he managed
to melt his flask to the heating element.
Ive never attempted the reaction myself but it looks like it requires some pretty high temps to drive the reaction forward.
At the end of the day though it could just be that this reaction is incompatible with primary amines.
Can i ask, what is your goal with this experiment?
Because if you are trying to get to an end product there are likely several other way to get there.
|
|
|