| Pages:
1
2
3
..
14 |
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
You can find all the information you want in Vogel's Textbook of Practical Organic Chemistry 5th Ed.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If you do your own scientific glassblowing or have access to a glassblower then this is pretty easy. The hard part is figuring out how to clean the
wire once if gets covered in carbon.
Also you had better have a fume hood. Ketene is very irritating, and is a known human carcinogen.
Also there are better sources of information than Vogel. Org.Syn. has better instructions for the building of a ketent lamp as well as alternate
ketene generating processes. Ace Glass used to sell such a lamp and still has a two page instruction sheet for it. Organic Reactions vol 3 has a
chapter on the ketene process and lamp construction details.
If you are only interested in ketene for making Ac2O there are other methods of making the anhydride that do not risk exposure to ketene. But in some
parts of the world the required reagents are hard to obtain.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A link to Vogel's page? Is this some kind of a joke?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Do you have a proper fume hood and other protective gear?
Because without those, making ketene is foolhardy and I for one do not wish to help you hurt or kill yourself or others.
This is not a game for the inept.
Start by learning what we mean by VOGEL.
VOGEL is free and it is on this website. I gave you a bunch of other sources, most of them also free, some of them on this website and others
elsewhere on the Internet.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ketene is a known human carcinogen. Not a suspected one, not a "rasonably anticipatred to be", but one of the few KNOWN human ones. It is also
extremely toxic and irritating and is comparable to phosgene in this regard. Merely doing this in open area is not enough.
What is your problem with the acetyl chloride + acetate method?
A ketene lamp at best generates about 0.4 mol ketene (and therefore, Ac2O if you bubble it into AcOH) per hour. You will need to make two or three of
the filament assemblies so you can swap one out while you clean the carbon off the other a couple of times per day. I have yet to work out a really
efficient way to do that. The literature talks about scraping the carbon off the nichrome wire, which strikes me as laborious and fiddly, likely to
result in broken filament.
There are also tube-furnace type ketent generators but these usually run about half as fast as the ketene lamp (if you want to call that fast.)
My advice is: work out your problem with the other method.
Forget about ketene for the sake of your health. Maybe your life.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
A bench scale ketene lamp will make at best 0.45 mol per hour.
A mol is the molecular weight in grams. Ac2O is MW 102 so the lamp will produce about 46 g per hour.
Those wattages are way to high.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
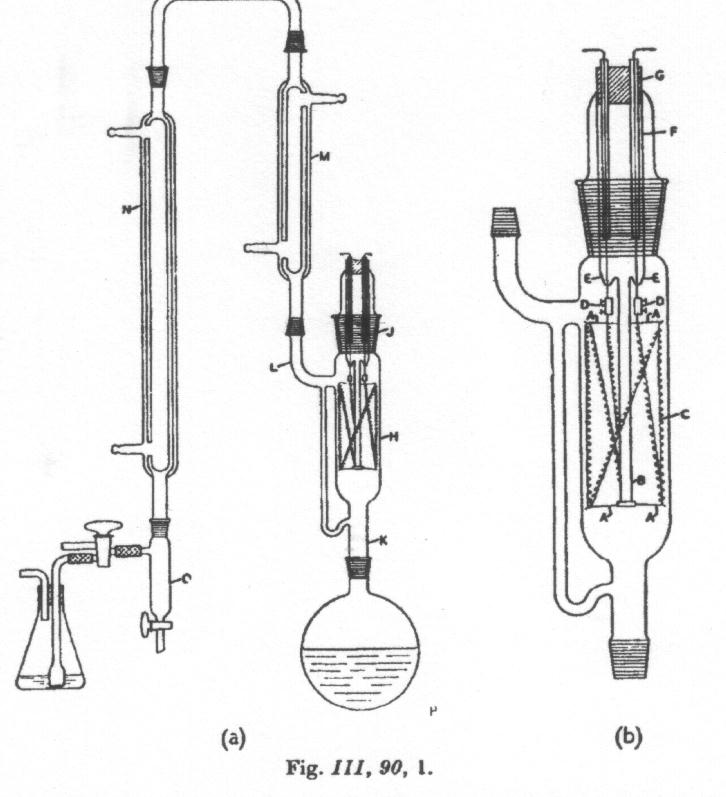
I will prepare a zip file of all the ketene generation material I have. And I will post it here.
The wire is usually a specific nichrome or monel alloy, specified, or else platinum, the latter being obviously expensive.
The wire is a common resistance type for heating elements, and the electricity needed is just enough to heat it to c.700 C. or less as specified in
articles.
You will need a competent scientific glassblower to fabricate this. No one makes these commercially any more although they used to. You might be able
to induce someone to make one for you custom.
I assembled this file as a pdf in case you don't have a archive file applet.
It contains
Chapter 3, Org.Reactions III
Vogel pp 372-374
Ace Glass instruction sheet for Ketene/Butadiene Lamp
A US patent
Two Org.Syn. articles
A journal article
You know have as much information as I do. Use it wisely, use it well, and don't hurt yourself or anyone else.
Download the file ketene.pdf here:
http://www.4shared.com/dir/2245331/5a78115f/sharing.html
[Edited on 20-3-2007 by Sauron]
[Edited on 20-3-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
1. For a tube furnace, tube can be Pyrex.
2. The nichrome does not participate in reaction. It's the heaet that does the work. One article recommends Platinum wire not nichrome.
3. The composition and diameter of the wire are important electrically, it is a resistance element.
Redistill your acetic anhydride using a fractionating column and take off product with correct b.p. only.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Balloon? A balloon has nothing to do with it.
I do not have any information about scaleup of ketene process to pilot plant scale. The various bench scale processes we have detailed for you will at
lest produce 500-1000 ml a day based on 24 hrs operation. I frankly do not think running this around the clock is advisable, and you are talking about
a scale 20-40X larger than that. That is the realm of chemical engineering my friend, and what do you need 20 L a day of Ac2O for anyway/ That does
not sound like amateur science to me.
Perhaps it is fortunate that you do not appear in any danger of success.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
A balloon flask is an alternative name for a round bottom flask, see this URL which has been mentioned repeatedly and look for balloon flask:
http://www.labxnews.com/labxnews279.htm

Chemistry is our Covalent Bond
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
He just said balloon nothing about flask. Had he said balloon flask I would have understood that to mean RB flask.
However my remarks still stand. Anyway flask size is not very germain, the limiting factor is the boilup rate of acetone that the system can handle.
Exceed that and all one gets is mostly unaltered acetone in the receiver.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
All right, @hector, sorry if I was a little cross. Are you acetylating wood upulp, or cotton?
I see there are processes for cotton using AcOH + H2SO4; and wood pulp using Acetic acid/Ac2O mixtures with H2SO4 catalyst. I wonder why acetyl
chloride can't be used? As you obviously can and must obtain it to make your Ac2O, and just as obviously either can't obtain the anhydride or else it
costs too much. And so you are attracted to the ketene process, despite its high risks.
The only document I gave you that describes anything scalable to 20 L/day is that US patent. The Org.Reactions Vol III article contains a lot of
references some of which might be to industrial processes. It seems to me that the tube-furnace process might be more easily scaled up than the
ketene-lamp. This despite somewhat lower efficiency on bench scale.
See if you can buy where you are, DIKETENE (ketene dimer) because that can be efficiently cracked back to ketene more efficiently than acetone etc.
can be pyrolyzed. However I don't know if this will be economical for you.
Can you replace your metal beakers with plastic ones or line the metal ones with teflon or glass to stop the reaction with acetyl chloride? Or you
might try decolorizing the anhydride with Norit (activated carbon powder) then filtration.
I will be happy to help (and I'm sure the other members will too) if we can solve your problems for you. The more we know about your process the more
chance we have to be of practical assustance. We don't get to advise commercial operations very much.
DMAP is 4-Dimethylaminopyridine. It is available in prilled form which is easier to handle (and safer) than other forms. Maybe DMAP will allow you to
eliminate Ac2O from your process, and use AcOH/H2SO4 instead. I don't know, it is only an idea.
------------------
I just read up on celluose acetate in Ullmann's. It seems that all of the bright ideas I listed above have been tried without success, including
direct acetylation of cellulose with ketene which I thought of but didn't suggest. Here attached is most of the Ullmann entry on this subject with
references (attached as text)
Of particular interest is the Wacker Chemical process for acetic anhydride from ketene and AcOH which is mentioned under recovery of acetic anhydride.
I will go look into this now.
[Edited on 22-3-2007 by Sauron]
Attachment: celluloseacetate.txt (32kB)
This file has been downloaded 3774 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It sounds as if your use falls into the hole between what is practicable on lab scale and on industrial scale. This usually is the range where you buy
reagents, rather than making them yourself, or buying the end product; the large manufactures can turn stuff out so much more cheaply than you can on
a small scale that it's just not economic to try to.
Industrially acetic anhydride is made via a ketene process, cracking acetic acid rather than acetone. Some is made by carbonylation of methyl acetate.
Much of the acetic anhydride produce is captive, being used ((often on-site) to make cellulose acetate. This usually uses a mixture of acetic acid
and anhydride with sulfuric acid catalyst.
The hot tube routes are the way to go for large scale, but as already been emphasised you are dealing with a rather toxic substance, phrases such as
Yokkaichi Asthma and Bhopal come to mind. The life you save is likely your own.
A monograph on acetic anhydride.
http://www.inchem.org/documents/sids/sids/108247.pdf
This may be of interest to you
Attachment: Process Flowsheeting Calculations for Acetic Anhydride Plant.pdf (73kB)
This file has been downloaded 9530 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
According to Ullmann's Encyclopedia of Chem.Technology (2003):
"Two other methods also were used in the past: the cleavage of ethylidene diacetate to form acetaldehyde and acetic anhydride in the presence of [NB
Sauron: Lewis] acid catalysts, such as zinc chloride, and the reaction of vinyl acetate with acetic acid on palladium(II) contacts to form
acetaldehyde and acetic anhydride [16]. Not one of these processes is now of any industrial importance."
If you can buy vinyl acetate or ethylidene diacetate -- both are acetylenic derivatives -- then these may be worth looking into. Vinyl acetate
CH2=CH-OAc Ethylidene diacetate H2C=C(OAc)2
I just looked up prices. Vinyl acetate is cheap and sold in volume (2.5L from Acros $33 US) Ethylidene diacetate is horribly expensive from same
source 100 g >$100 US. So I would focus on vinyl acetate. Warning: flash point -8 C.
Let me look at reference 16 and see what this process looks like but this one just might be of use to you.
Well ref 16 is Ullmann's 3rd ed, Vol 6, p.804. Which I do not have. Can ahnyone help?
[------------
I am on the trail of this now. Acetylation of sec. alcohols with vinyl acetate over PdCl2/CuCl2 is a known reaction with acetaldehyde as byproduct so
it seems like in absence of an alcohol to acetylate, the actual acetylating agent (Ac2O ?) will be isolable. Therefore the system PdCl2/CuCl2 seems to
be the catalyst. I am ferreting out details. This could be a winner, easily scaled to the 20 L/day Ac2O that is desired, and with fillip of
acetaldehyde byproduct readily oxidized to AcOH which @hector2000 can utilize as well.
Got it! Clement and Selwitz, Tet.Lett.1962, 1081. PdCl2 catalyzes conversion of vinyl acetate into acetaldehyde and acetic anhydride in
glacial acetic acid containing sodium acetate. .
55% of vinyl acetate is catalytically hydrolyzed to equal amounts of acetaldehyde and acetic anhydride under mild conditions and rapidly. The
experimental conditions employed 2% molar ratio of PdCl2 (0.005 mol) for vinyl acetate (0.1 mol) in 60 ml glacial acetic acid stirred 1 hr at 40-80 C.
producing 0.048 mol acetaldehyde and 0.05 mol acetic anhydride.
I think we have a ball game here.
[Edited on 23-3-2007 by Sauron]
Attachment: Palladium chloride catalyzed decomposition of vinyl acetate in acetic acid [1].pdf (228kB)
This file has been downloaded 1472 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The palladium is recoverable and reusable. You want to use acetylene, if you're doing that route to the anhydride. Ketene reacts directly with water
to give acetic acid and with acetic acid to give acetic anhydride, you don't need any other reagents.
I'm pretty sure ketene and HCl give acetyl chloride, but why? If you want it to react with sodium acetate, ketene plus acetic acid gives the anhydride
in fewer steps with higher yield and easier workup.
Again, one industrial process is effectively ketene + acetic acid => anhydride, the ketene being form in the gases by dehydration over an AlPO4
catalyst.
The references I've seen for making cellulose acetate all use a mixture of acetic acid and acetic anhydride, with a bit of sulfuric acid. There
doesn't seem to be a great need to isolate the anhydride. The cellulose can even be acetylated by suspended the cellulose in acetic acid and passing
in ketene.
Why not just buy the anhydride, or even the cellulose acetate? The manufactures of it have spend decades optimizing their acetic anhydride production,
and do it on a huge scale. There's no way you can make acetic anhydride as cheaply, efficiently, and as pure as they can. Likewise they've got their
reagent recovery down to a 'T', they waste as little of the raw materials as possible so their cellulose acetate is likely to be cheaper than you can
make it.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
After the reaction, you will have a mixture of acetaldehyde, acetic anhydride, unreacted vinyl acetate (to recover and recycle), acetic acid (recover
and recycle) sodium acetate (recover and recycle) and PdCl2 catalyst (recover and recycle)
The PdCl2 is a heterogenous catalyst, it is insoluble in that mixture so first thing you do is filter the mixture to remove the PdCl2 (or perhaps it
is Pd metal by then, I am not sure.) In any case you should be able to recover it quantitatively. That means you only have to buy it once. If it
remains as PdCl2 then you just wash it and reuse it. If it has become Pd metal you must digest it in HCl and recover it as PdCl2.
So now you have AcOH, Ac2O, acetaldehyde and vinyl acetate and some sodium acetate. The acetaldehyde is quite low boiling (21 C) and will distill off
first. My advice would be to oxidize it to acetic acid which you can use anyway as you have no use for the aldehyde that I know of.
For the rest let's look at boiling points.
Vinyl acetate 72-73 C
Acetic anhydride 140 C
Acetic acid (glacial) 117-118 C
So the unreacted vinyl acetate will come over second, now all you have left is AcOH and Ac2) and sodium acetate.
Now you don't want to have to distill off all that acetic acid and you would rather remove the anhydride instead. Sounds like a job for liquid-liquid
extraction. Let's look for a solvent that will extract the relatively small amount of anhydride from the larger amount of acid without dissolving the
acid. If you can do this you don't need to distill the acetic acid at all, or seperate the sodium acetate, you can just use them in the next run.
@n_i, we are onto a completely different process here. The ketene process is too dangerous and in fact is obsolescent since the mid 70s because of the
high energy costs (truer now than then!) but lingers on because of the high cap.investment in those plants. The Halcon process and catalytic oxidation
of acetaldehyde through peracetic acid is not downsizable. So we are looking at catalytic hydrolysis of vinyl acetate w/PdCl2 in AcOH instead. This
liquid phase, at atmospheric pressure, decent yield and so we are looking at the workup.
If you go look at the acetic anhydride thread I found another prep, dry reaction of sodium acetate with N2O4 gives Ac2O and NaNO2 and N2O3 (Balanced
eq is there not here.) However I don't think our friend @hector2000 wants to get up close and personal with enough N2O4 to make 20 L a day anhydride,
he might as well be breathing ketene or phosgene. Also I have few details on this 1930s Russian process.
[Edited on 23-3-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Acetaldehyde is far far LESS irritating than acetic anhydride and even less than ketene which is deadly. Ketene if it doesn't kill you now will kill
you later (carcinogen) and there just isn't any getting away from this simple fact. So unless your factory is in the middle of nowhere this will be a
danger not only to you and your staff but also your neighbors (remember Bhopal? Ketene is a lot worse than methyl isocyanate.)
Vinyl acetate is higher boiling than acetaldehyde but has a much lower flash point. For that matter acetic anhydride is also very flammable.
Since the vinyl acetate hydrolysis is run at 40-80 C and acetaldehyde boils at 21 C obviously you can cimply take off and condense acetaldehyde as it
is formed which will be easier than refluxing it which would require recirculating chillers at <0 C or maybe Dewar condensers with freezing
mixtures.
Maybe you can reduce the amount of acetic acid solvent and you can determine this by smaller scale trial and error.
I think you will find that you can achieve certain economies with the PdCl2 because you can reuse it from batch to batch with essentially no losses.
Let me make some calculations. I will work backwards from 20 L a day anhydride and see what batch sizes, reactor sizes and number of batches/day pop
out.
----
Meanwhile you have a working process (AcCl + Na acetate) but it is giving you problems, let's look at your problems. Supplier of acetyl chloride is an
idiot using improper container and contaminating reagent with FeCl2, correct? Is changing to another supplier an option? Or can you convince present
supplier to package the acetyl chloride properly so to eliminate the iron salt problem?
Can you purchase cyanuric chloride? This is cheap. Reflux 1 part of CC with large excess of AcOH and you get 3 parts acetyl chloride in there which
you can strip off the acetic acid easily as it boils low. (By product cyanuric acid ppts out, filer it off, resuse acetic acid with more CC.) This
way you make your own acetyl chloride and reduce costs while eliminating FeCl2 problem.
A little more expensive, you can buy oxalyl chloride and make acetic anhydride from acetic acid directly with it.
Or you can make the oxalyl chloride from oxalic acid and CC then use the oxalyl chloride to make acetic anhydride. In these last two methods you
bypass acetyl chloride completely.
You haven't told us why you can't just buy acetic anhydride, but I assume you can't. In this country I can't buy either anhydride or acetyl chloride
so I must make my own but I don't need 20 L a day! Maybe 5 L a year.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Of course acetic anhydride is highly restricted here for same stupid reason. But I am sure they make cellulose acetate here and so they get a license
to import from the Defense Ministry who control this. Acetyl chloride also. And many other things.
CC is solid, it smells but it is not dangerous to handle it, I have many Kg in my home. In EU they consider it toxic but they consider lots of things
toxic because of the Green politics. There's a difference between pretend toxic and TOXIC. Ketene is REALLY TOXIC. So my advice is to stear clear of
that process.
We will find you a way to get this done that won't kill you and won't bankrupt you.
I understand your problem.
The acetyl chloride, is it already contaminated before you open the bottles or does it only become contaminated (yellow) later?
Have you tried decolorizing it with powdered activated carbon like Norit? Just put in a few grams per liter, stir, filter, color should go away
(trapped in the carbon). You would want to do this in closed flasks otherwise the acetyl chloride will fume badly and stink up the place. It is more
irritating than acetaldehyde.
If we solve this problem with acetyl chloride then you will at least have a good process till you can find a better/cheaper methed with or without our
help.
Another member has suggested a US patent that says you can make Ac2) from vinyl acetate without PdCl2, I am investigating.
And I am still looking into how to get the Ac2O out of the acetic acid without distillation.
The other name for CC is TCT and it is used to make agrticultural chemicals, it is cheap. In fact I have seen articles about it by chemists at
universities in Iran so it ought to be available in your country maybe manufactured there. I will get the full name for you and the CAS number. The
name is trichloro-s-triazine, or cyanuric chloride.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
You can't use cellulose acetate molding powder from the plastics industry?

|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@hector 2000 already explained that in his country cellulose acetate is $30/Kg, and he is making his own to save cost. If he cannot solve problem of
better way to make his own Ac2O he will have to close his company. He needs to make 20 L a day anhydride to produce 50 Kg/day cellulose acetate. He is
presently making Ac2O from acetyl chloride and sodium acetate but the acetyl chloride he can buy (locally made apparently) is packeged badly and
contaminated with FeCl2 so is yellow. The good news is, it is cheap.
@hector, are you fusing the sodium acetate prior to reaction? It needs to be totally anhydrous, so you need to melt it in a drying oven at 125 C then
break it up and powder it, all this just before using it, or you will get less acetic anhydride because part of the acetyl chloride will be used up
reacting with the water of crystallization. This also true of sodium acetate in the vinyl acetate method.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I suspect your supplier is making acetyl chloride by POCl3 or PCl3 and not purifying so still contains some phosphorus compounds. Or could be sulfur
compounds from SOCl2. If I were you I would distill the acetyl chloride before using and bottle it in amber (brown) glass bottles only with corrosion
resistant caps and store it away from light in a cool place. Obviously the acetyl chloride is passing the P or S contamination along to the acetic
anhydride and this is source of your problems.
CC (TCT) is CAS registry number 108-77-0. Name 2,4,6-trichloro-s-triazine. If you make acetyl chloride from this it will never turn yellow or red. But
store it properly cool and dark in brown glass and don't use a cap it will attack.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron..,
@n_i, we are onto a completely different process here. The ketene process is too dangerous and in fact is obsolescent since the mid 70s because of the
high energy costs (truer now than then!) but lingers on because of the high cap.investment in those plants. The Halcon process and catalytic oxidation
of acetaldehyde through peracetic acid is not downsizable. So we are looking at catalytic hydrolysis of vinyl acetate w/PdCl2 in AcOH instead. This
liquid phase, at atmospheric pressure, decent yield and so we are looking at the workup.
...
[Edited on 23-3-2007 by Sauron] |
At the time I replied you'd moved on, but
| Quote: | Originally posted by hector2000
what is carbonylation of methyl acetate method?
is method better than keten method?
and may i replace the acetylen gas with keten?
and another question:
HCL+KETEN->ACETYLE CHLORIDE
this reaction may be true?
[Edited on 22-3-2007 by hector2000] |
which meant he was still thinking about ketene for one reason or another.
The vinyl acetate method is related to the older acetylene + acetic acid => ethylidene diacetate => acetic acid & acetaldehyde. As vinyl
acetate can be (& was) made by adding acetic acid to acetylene, all that process you found does is effect put an extra step in. This could be
handy as you point out vinyl acetate is a commodity and easier to fling about than acetylene; on the other hand vinyl acetate will contain
polymerisation inhibitors that might cause problems (distill the VA into the reaction mix?).
The point here is that process goes back to somewhere in the 1920s, it's mentioned in J.B. Conant's Chemistry of Organic Compounds, a
textbook published in 1934. Finding some industrial references from that period could help with the workup. Same book has anhydride being made with
S2Cl2 and sodium acetate. Alas, no references for either method, it's just a textbook.
BTW - ethylidene diacetate can be split into vinyl acetate and acetic acid, and it can be made from acetaldehyde and acetic anhydride; the reverse of
the reaction you've been researching, and used in the US around mid-century.
Could hector transfer the acetyl chloride into all glass temporary storage immediately upon opening the container? If the color problem is iron
introduced by corrosion this might help.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@n_i, if you look carefully I mentioned ethylidene diacetate -> acetaldehyde + acetic anhydride but I also checked the price and ethylidene
diacetate is about 100 times more expensive than vinyl acetate so I dropped it like a hot rock. Vinyl acetate is cheap. Acros sells 2.5 L for $30.
Largest vottle of ethylidene diacetate is 100 g # $122. Furthermore, although anyone who has googled the subject would know that in essence this is a
reversible reaction, and acetaldehyde + acetic anhydride is catalyzed by Pd(II) to vinyl acetate, if you read the article from JOC by the two chaps
from Gulf (the Seven Sisters) you will see that the particular reaction conditions at hand do not proceed through ethylidene diacetate and that in
fact that substance is entirely unreactive under those conditions. Therefore ethylidene diacetate merely muddies the water.
Thanks for your suggestion regarding rebottling the acetyl chloride but, again, read what I already posted and you will see I did think of that and
already suggested it as a remedy. According to @hector2000's latest, the acetyl chloride is Merck product. This is really odd, Merck ought to know
better than to package something in such a fashion, wouldn't you think? Also while yellow sounds like FeCl2, red does not and this stuff goes red, and
the Ac2) made from it also goes first yellow then red. This is most odd.
@hector2000, I have in the past used Russian method which is anhydrous sodium acetate plus sulfur and bromine to make acetic anhydride. Presently I
have purchased CC so I can make oxalyl chloride and with that make either acetyl chloride or acetic anhydride as needed. Also I am buying oxalyl
chloride as well but it is not cheap. Better to make it from cheap CC.
I have no experience with the persulfate reaction but I know that some European members are looking at it because they cannot easily buy CC. I don't
have this handicap, so I have not examined this reaction carefully. For me CC is easy and cheap and versatile. I can make with it acid chlorides, acid
bromides and iodides, alkyl chlorides, and particularly oxalyl chloride. With that I can make anhydrides directly. Also inorganic acid chlorides that
are hard to buy here. CC is also useful for a number of other transformations.
So far I have found only one solvent that acetic acid is insoluble in, carbon disulfide. I am not yet certain whether or not it dissolves acetic
anhydride. The main difference between the acid and the anhydride is that the former is protic and the latter is not. We can exploit that. I am still
investigating. CS2 is a little too flammable for my liking, you will already have enough problems with vinyl acetate. I will report as soon as I have
news.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am first to concede I am not a chemical engineer. On the other hand, making 20 L or 50 L a day is NOT industrial scale. On industrial scale things
are usually done on a continuous basis not on batch scale (whenever possible) and generally are automated. You don't want or need any of that to make
50 L a day or 20 L a day, you are not working round the clock and you are working in batches not continuous processes. So you are working in a large
bench scale.
I'm a retired guy not a student. It has been a long time since I was a student. When I was a student Reza Pahlavi was still running your country, if
that puts things in perspective. However I never stopped studying...
Here's a tip. If you have to filter something unpleasant like acetyl chloride and you don't want to have to deal with the fumes from a open top
Buchner funnel, you set up a pressure filtration appartatus and you use air or inert gas (as appropriate) to move the liquid through tubing to a
closed fritted disk funnel. You must make sure your gas pressure does not exceed the mechanical strength of the fritted disk.
This is similar to moving air sensitive reagents from bottle to reactors.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
The separation of acetic anhydride from acetic acid is rather simple if one has the proper equipment 
Attachment: US5264087A1.pdf (197kB)
This file has been downloaded 1723 times
Chemistry is our Covalent Bond
|
|
|
| Pages:
1
2
3
..
14 |