Odd "plasticy" crystals while making Copper Acetate
Decided to try to make some copper acetate with vinegar/H2O2 solution that is 2.5%/2.5% . I filled a large funnel with small pieces (1/4 - 1/2" long)
22g wire pieces - about 8 - 10 lbs and placed some cotton balls in the bottom of the funnel to filter & hold the wire pieces. The wires had black
copper oxide on the outside from being burnt (washed well before using). I dripped the acid solution with a sep funnel at about 1 drop a second.
after about 250ml I had tons of foam (3" deep & 8" diameter funnel) and it the copper was pretty warm and a lot of CuO was floating on top of the
foam. slowly poured 5% vinegar on the foam, it slowly dissolved bubbles. Recovered about 400 ml of beautiful blue clean clear solution under the
funnel. It looked like a very clean reaction product.
I evap'ed about 200ml in 2 dishes (oven at 230-240F) and allowed the other 200ml to evap at room temp. I got very odd reactions with all of them. I
then used the same copper and just vinegar boiled on stove - about 3L, filtered, boiled down solution and got the best acetate crystals I've ever had.
Small but perfect in color and consistency. Did the same with vinegar & H2O2, filtered & boiled down and got same nice crystals. So it seems
the acids/H2O2 and Cu are all fine and make expected product.
Now the pics of the first solution.
This is the petri dish from slow drip then evap'd at ~235F

Same as above but closer pic

Same batch as above but larger dish with more solution. Looks like CuO on the top which is odd b/c copper acetate shouldn't have decomped anywhere
near that temp and it wasn't under direct heat.

Same as above but without the burnt layer on top. Not much crystals and it is an odd compact "dust".

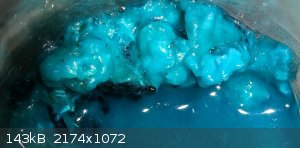
This is from the same solution as above but evap'd at room temp for 2-3 weeks. Notice the light color "fluff" that is very light blue/white-ish. IDK
how this formed and there are normal acetate crystals in it as well (see around top right edge). The white-ish is very light and it does dissolve in
water. I am wondering if the peracetic acid or copper acetate (or the mix) might have dissolved the cotton and this is a result of it?

Closer pics of above

Closer pics of above
|