| Pages:
1
2 |
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Colour of potassium nitrite
I made potassium nitrite yesterday and posted that video on my channel .
If you will see the video you will observe that the filtered solution of potassium nitrite was slight yellow in colour.
Is it due to the tar contained in charcoal or is it the characteristic colour of potassium nitrite?
And won't tar be burnt in that reaction . Plz see the video carefully and answer this question .
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The yellow tint is normal for alkali metal nitrites as a quick glance at their wiki would've told you had you bothered to look...
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
this would be a case to add a simple link to the video in question, or a link to your channel on signiture.
As stated when making a substance it is your duty to research both starting materials and their properties, and the expected target substances
properties.
In the case of your research you'd have easily found your answer.
Yes, a slight yellow tinge is what they tend to look like.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
I know that wikipedia says that potassium nitrite is slight yellow in colour . And I have also seen sodium nitrite in lab it was also slight yellow in
colour . I want to ask someone who has worked before with nitrites and has a good experience of it
|
|
|
Texium
|
Thread Moved
19-3-2018 at 04:12 |
Texium
Administrator
       
Posts: 4676
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Sounds more like you're fishing for views
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Stop the fishing dude, I hope you don't need more explanation about why. Also; it doesn't work, everyone here knows what you are trying to do.
My potassium nitrite is completely colorless.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
I am not fishing views over here . Enough you have spoken . Enough is enough . It's too much now.
Even the lab grade sodium nitrite is slightly yelloe and you say it is colourless. I think many people have problem with my posts because they are not
interested in that topic .
You can only see that I am fishing views over here but cannot see that first of all I am posting videos for nothing and giving correct information .
People cannot see other people getting benefit and I think you are one of them
And if I am sharing my videos or my creations with my SM members I don't think that's fishing . I think that's sharing of ideas on a correct platform.
The people who are interested will like it . The people who are not interested will ignore it .
[Edited on 19-3-2018 by Akhil jain]
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I will take a picture of mine tonight.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The video/yellow colour is this one: https://youtu.be/9J9Rlz5xahI?t=245
My nitrite was prepared using the classic lead reduction method :
http://www.sciencemadness.org/talk/viewthread.php?tid=52&...
The yellow tint is almost impossible to see unless you sneak up on it and take a peek while it's back is turned.

On the left is some sodium nitrate, probably prepared with nitric acid & some sodium salt (can't remember).
On the right is the sodium nitrite prepared a couple of years back, using some of the same nitrate seen on the left.
@Akhil: if you're convinced there's no Fe present, try the activated carbon trick to see if it clears that yellow solution up a little.
With the best will in the world, a straight combustion-in-air reaction is going to generate a hell of a lot of side-products, no matter how well you
calculate the stoichimetry.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Erm, that's bending the fabric of Reality further than even my brain can imagine after 16 pints of strong lager.
"... the Correct method to prepare potassium nitrite ..." for example.
I guess H.T. Vulte never imagined just setting fire to stuff when he wrote his book "Laboratory Manual of Inorganic Preparations" back in 1895.
Edit:
Seeing as simply burning KNO3 + C does produce some KNO2, it might be worthwhile heating that mixture in a stream of
argon (to remove the CO/CO2 as it is produced).
Might be a much better way than using lead.
[Edited on 19-3-2018 by aga]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Might it be just the products of side reactions burning charcoal? how pure was your charcoal..?
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
OK, I actually watched your video and I'm pretty certain the yellow color is due to the charcoal.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Guys my charcoal was simple charcoal it was not lab grade charcoal . And I would try clearing it by activated charcoal but I don't have activated
charcoal .
Does anyone know how to activate simple charcoal.
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by aga  | The video/yellow colour is this one: https://youtu.be/9J9Rlz5xahI?t=245
My nitrite was prepared using the classic lead reduction method :
http://www.sciencemadness.org/talk/viewthread.php?tid=52&...
The yellow tint is almost impossible to see unless you sneak up on it and take a peek while it's back is turned.
On the left is some sodium nitrate, probably prepared with nitric acid & some sodium salt (can't remember).
On the right is the sodium nitrite prepared a couple of years back, using some of the same nitrate seen on the left.
@Akhil: if you're convinced there's no Fe present, try the activated carbon trick to see if it clears that yellow solution up a little.
With the best will in the world, a straight combustion-in-air reaction is going to generate a hell of a lot of side-products, no matter how well you
calculate the stoichimetry. |
From my experience working with bulk NaNO2 (above 1 pound or .45 kg), I can confirm that there absolutely is a yellow color. Mine is
technical grade, but the only impurity that I know of is an anti-caking agent (insoluble anyways). The solid takes on a slight yellow color, even when
cracking open the bottle for the 1st time. Even the water solution of it takes on a pale yellow color.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yay !
My amateur chemfumbling made a better grade than Technical !
ISTR it was a lot of work.
https://www.sciencemadness.org/whisper/viewthread.php?tid=63...
Edit:
OMG ! Just re-read that thread and i pyrolysed some bread even back then !
Rough (but experienced) stab at a way to make Great Activated Carbon :-
Pyrolyse some bread.
Stick it in a tub of water
Freeze it
Thaw it out.
Ball-mill it.
Chances are that it makes an A.C. with an I.N. of over 1,000.
Maybe repeating the freeze-thaw-ball-mill part will get it way up there.
Deffo got to try that one.
[Edited on 19-3-2018 by aga]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
The food grade sodium nitrite I have is slight yellow in colour. Since both K+ and Na+ are colourless, I’d presume it would be the same either way.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Please post a photo for comparison.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
That's a so long discussion aga on AC
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sorry it was not simple.
Edit:
Condensed version :-
Pyrolyse some bread.
Stick it in a tub of water
Freeze it
Thaw it out.
Grind it up.
(maybe grind before freezing)
[Edited on 19-3-2018 by aga]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Only got about 50-60 grams left, gotta get some more soon.
Apparently it’s 99.9% pure, it’ll have to be high for food use but in any case it’ll be at least 98%.

[Edited on 19-3-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks LearnedAmateur !
That yellow stuff looks highly granular, like cubic crystals.
Very much like the wiki image shows.
I guess mine is more white and fluffy due to idiocy and/or Pb salt contamination.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Yeah it’s got the consistency of table sugar as far as I can see, just a little smaller. Time to bust out the ghetto microscope for a better look!
Sucks though because it doesn’t exactly show true colour and it gets in the way of the flash, but I’ve got some cool pictures with it before.
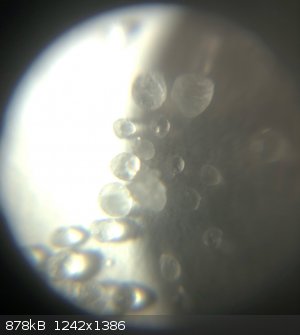
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
There are references that say nitrite is white to yellow, but most just say slightly yellow or yellow white. I think white to yellow actually means
somewhere in between. All of either cation that I've seen is the same color, like the ~yellow of the site. Same as a few pounds of food grade on hand.
(the color is best viewed with, or on, something white)
BTW aga there is a difference between what prepchem says Biltz said on the reduction of nitrate, and what he actually wrote.
(explore your links and my www button)
[Edited on 20-3-2018 by S.C. Wack]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by S.C. Wack  | | ... there is a difference between what prepchem says Biltz said on the reduction of nitrate, and what he actually wrote. |
Damn.
Wish i could find that stuff.
Could there be a reference ? Who's Biltz ?
|
|
|
Texium
Administrator
       
Posts: 4676
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
My sodium nitrite (I think it's reagent grade but I can't remember- it was a group-buy thing) is just barely yellow enough to notice- less yellow than
the picture that LearnedAmateur posted. Perhaps the differences in color are just due to differences in crystal size and/or hydration.
[Edited on 3-19-2018 by Texium (zts16)]
|
|
|
| Pages:
1
2 |