Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Practical chemistry and common ion effect
Mant times I have tried this experiment but every time I get a precipitate. I take some zinc chloride soln. in a test tube ,add NH4Cl in it and then
add NH4OH but always i get a ppt and according to this book Zinc ions should not ppt due to common ion effect and their higher solubility product .
Same thing is happening with manganese instead of precipitating in group 4 they get ppt in group 3 .
Guys please try this and tell is same happening with you
These are photos of that book . You may understand from this what I want to say
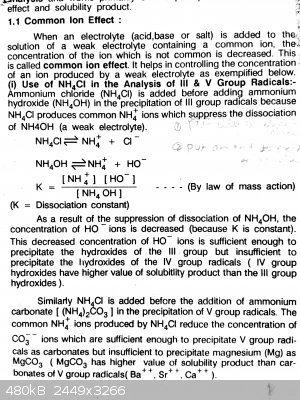 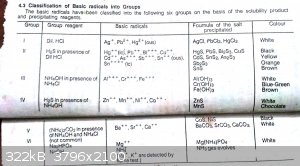
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Zinc hydroxide is insoluble, but in excess ammonia it dissolves to form an ammine complex. If you want it to dissolve, try adding more ammonia.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
I know that it dissolves in excess ammonia but what about the analysis .you aren't getting my point . What about common ion effect
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Plz try the experiment and tell me did the same happened with you
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
You need to add more ammonium chloride if you want to see the common ion effect in action.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Have you tried
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Ya bro you were right . I tried and this time I could not get a ppt with zinc ions but got a ppt with Fe2+ ions. Why did they get precipitated their
solubility product is almost of same order of other ions of group 4
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Because zinc forms a complex ion with ammonia, and iron doesn't.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Then what about common ion effect
|
|
|