RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Convection for high temperature
Can high temperatures (at least 2000 degree celsius) ever be made without any additional helper like bellows or fans or chemicals?
What parameters of chimney or pipe play important role?
What affects draft practically?
Is it length of pipe/chimney that determine strength of draft and therefore the tempearture furnace can make?
Is narrow better than wide chimney?
If wood or any other natural fuel is burned.
Is geometry (look) of chimney important. Pipe or chimney normally is same as letter I or l. Should it be changed to shape similar to A (chimney
narrowed towards top) or V (chimney narrowed towards bottom)?
As far as I have noticed, blast furnaces are narrowed towards top? Is there some temperature-affecting reason or just to not lose more material for
making chimney?
I am talking about natural draft or convection.
[Edited on 11-2-2018 by RawWork]
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
According to this:
https://en.wikipedia.org/wiki/Gas_burner
...the temperature of a wood flame is just short of 2,000 C at most. I believe to effectively heat a reaction chamber or the like to 2,000 C you
would probably need an oxygen-fed gas flame. (They list acetylene burning in air as being hot enough, but an acetylene-air flame can be very dirty
and unpleasant to work with since the acetylene tends to polymerize if oxygen-starved at all, creating nasty smoke and soot.)
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Isn't flame dirtiness, color, smokeness only relative? It can be avoided by supplying more air at some part of pipe, like in bunsen burner at bottom
to change flame color and temperature. Isn't temperature relative? Meaning what they say for that maximum temperature, it's only in standard
conditions, in air? Would not stronger draft overcome that limit for wood, coal, gases? I don't intend to use any other fuel than wood for making high
temperature, at least in my current situation. I have a lot of wood.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
2000°C is extremely hot. 1200-1300°C is white heat, I believe. I don't run my tube furnace over 1000°C, a yellow heat.
If you stay with wood first dry it well. Wood out of the forest is 50% water. Then, using a ceramic furnace pump in pure oxygen. That should get
you there.
ZrSiO4, Al2O3, MgO, and mullite come to mind as suitable materials of construction for your furnace.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
I don't have pure oxygen. Making high temperature using oxygen only and fans is easy. Finding fan would be much easier than finding pure oxygen, if
one of these two is a must have for high temperatures.
[Edited on 11-2-2018 by RawWork]
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I believe that the heat radiated and/or conducted away will be so great that 2000oC is impractical using wood + air.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | | I believe that the heat radiated and/or conducted away will be so great that 2000oC is impractical using wood + air.
|
Even if fans are used with extreme speed?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
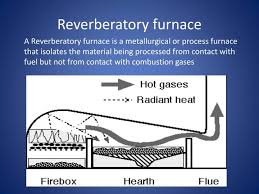
If you have lots of wood and want maximum heat for a process, converting the wood to charcoal is often done. The off gassing material from pyrolysis
of wood can also be used as a fuel in a reverbatory furnace, where the heating of the process material is partially acomplished by RADIATION from the
flame and the heated furnace ceiling, as opposed to purely from convection, this may allow higher process temperatures.
Part of the limitation of temperatures available from naturally aspirated burning is the large ammount of cold Nitrogen which comes along with the
Oxygen. It doesn't help oxidize, but DOES carry process heat away with it up the chimney-
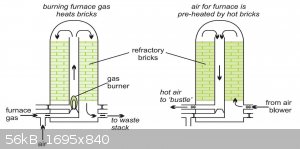
A way to partially counteract the heat loss from the inert Nitrogen gas load is to pre heat your combustion air. In a blast furnace, there may be two
"checker chambers", which are alternately heated with the exhaust gasses passing out of the furnace and used to pre heat combustion air-

[Edited on 11-2-2018 by Bert]
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
That sounds practical. Luckily I am not worried about efficiency or losses of anything. I only care about making that high temperature, even if many
steps and losses are neccessary. What worries me a bit is a theory. Is it superstition that preheating air will increase temperature of charcoal
burning? Is it superstition that using exhaust gases to heat something will improve anything? First thing decreases density of air. Second thing
reduces convection, block air somewhat. Does these two things reduce or increase temperature overally? They are against each other.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
You need to learn basic thermodynamics and fluid dynamics theory and how to use math to check your theories, then you can start answering questions
like this, then pick a likely scenario to do an experiment and verify.
Overview of thermodynamics
Fluid Mechanics, Modeling & Measurement of Gas Flow
I have seen a small blast furnace home built in someone's back yard. Kind of surprising... Experiments are possible.
Really basic, bloomery
https://youtu.be/W6uFAv9L734
Or more sophisticated, producing liquid Iron
https://youtu.be/UXeCYmAQg6U
[Edited on 11-2-2018 by Bert]
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Isn't flame dirtiness, color, smokeness only relative? It can be avoided by supplying more air at some part of pipe, like in bunsen burner at bottom
to change flame color and temperature. |
Yes, certainly it's a matter of a good mix of fuel and oxygen. In my experience acetylene is particularly intolerant of shortcomings in the air mix
in terms of keeping it burning cleanly, but it might be worth trying. Acetylene seems like the only practical option if you're looking for a very
hot flame without needing an oxygen cylinder.
The idea of pre-heating air in one furnace in order to feed it into another furnace is an interesting one, and might work if carefully designed and
tended to. I would probably feed in air with a fan at one end and use a damper to control the flow of heated air into the second furnace.
Insulation would be important. Construction materials could be a challenge since 2000 C will melt steel.
I'm guessing you're thinking of working at a relatively large scale (production rather than experiments)? In most areas a cylinder of oxygen and a
regulator won't cost much, but replacing cylinders constantly would be an expensive way to power a production-scale process.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Pure oxygen can be obtained in canisters at the hardware or welding supplies store.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
If you are purely looking for a source of high temperature
then a carbon arc may be feasible.
Especially if you have access to arc welding power supply.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
semiconductive
Hazard to Others
  
Posts: 325
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by RawWork  | Can high temperatures (at least 2000 degree celsius) ever be made without any additional helper like bellows or fans or chemicals?
[Edited on 11-2-2018 by RawWork] |
Yes, quite easily if you don't burn the fuel .. but use Electricity inside a very small sealed chamber. You could even make a furnace box with only a
steel plate in the bottom, lay it on top of about an inch of refractory, and then place the whole box on top of an modern kitchen induction stove. (I
wouldn't recommend it, the stove's expensive if you screw up.)
But ... I htink you want something really cheap, if possible?
Very old 1950's style electric furnaces were made with simple graphite and pitch heating elements. Lubricating graphite (pencil lead graphite, too),
gets very hot when electricity is run through it. Sometimes a mixture of clay, graphite and charcoal were used as a heating element. I'm not sure of
the exact proportions, but the only major trick is to exclude air to keep the charcoal and graphite from catching fire.
I did an experiment once with 0.5mm pencil lead and put it under oil (no air) with two tungsten contacts on the end. I was counting on leidenfrost
effect to allow it to operate for a few seconds before the oil would char. Under high voltage, the pencil lead glowed white hot for about 15 seconds
before the oil became so charred you couldn't see the light any-more. The grapite stick was so small that it didn't have much thermal mass, so this
isn't practical for a furnace -- it just shows that graphite can easily get above 1300C without melting or breaking. The limit for graphite use is
something like 3800C. It's the materials AROUND the graphite which need to be resistant to reducing atmosphere and extreme heat.
So, as long as you can figure a way to prevent air from getting to carbon/graphite ... that's an excellent and cheap material to get the job done that
you want. I thought about using molten metal as an air shield ... ahven't tested it yet, but common, easy to work with, and cheap substances usable
as part of a heating element for 2000C furnace would be tin, graphite, and especially silicon carbide (AKA valve grinding compound for car engines);
If you don't want to mess around, just buy a molybdenum wire or commercial heating element. It costs, but the stuff is a champion in high heat
applications.
Silicon carbide is easy to get electrical semi-conductor, it's also a very good heat conductor which important for the heating element's surface ...
but unfortunately silicon carbide is hard to find with low enough resistance to use in a home. But ... you can still use it as a part of the
refractory protecting a cheap molten heating element.
For the heating element itself, you only really need to a cheap metal (disposable after a number of days of use) that won't boil below 2100C. eg:
somthing like iron or especially tin.
I would think a mixture of things like steel wool, tin, graphite, and silicon carbide grinding powder would work. You would need to put it in a
carved trough made of refractory (like silicon carbide) that can take 2000C.
I plan on making a carbon silicon heating element this summer using a special technique. I've destroyed several canthal elements, and they're too
expensive for the short lifespan and accidents.
The only bane of this type of cheap furnace is that the refractory can't crack, or else the heating element will be cracked and if the crack is wide
enough, the liquid metal will sink and the element will break.
|
|
|