Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Dehydrating SnCl4 * 5H2O
Hi all,
Just looking for an easy way to dehydrate Stannic Chloride pentahydrate to the anhydrous form. I made the pentahydrate by combining solutions of
Stannous chloride and Sodium Nitrite, which gave the precipitate. Suggestions are welcome.
Thanks,
-R/T
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Use a desiccator or if you have one, a Dean-Stark trap. I don't think there is an easy way to dehydrate it quickly, as boiling down and evaporation
would cause a significant loss in product.
The philosophy of one century is the common sense of the next.
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Convert it to Potassium Hexachlorostannate and distill.
"Sodium Stannichloride, Na2SnCl6.5H2O, crystallises in prisms when the concentrated solutions of the constituent salts are mixed together, the
potassium salt K2SnCl6 crystallises in anhydrous regular octahedra, with which the ammonium salt (NH4)2SnCl6 is isomorphous. This latter salt was
formerly used by dyers, and was named pink salt, because of its use as a mordant for madder-red colours; it dissolves in 3 parts of water at 14.5°
C., and from its dilute solution stannic hydroxide separates on boiling. A large number of stannichlorides have been prepared containing various
amounts of water of crystallisation; the chlorides of barium, cadmium, copper, silver, lead, and thallium, however, appear not to combine with stannic
chloride." From atomistry.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I do not think that anhydrous SnCl4 can easily be obtained from the pentahydrate,
you may be able to get to the dihydrate but I guess less hydrated would be quite difficult as SnCl4 is decomposed by hot water.
I'll let those more knowledgable comment.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Sulaiman is quite correct. There is no practical way to convert the pentahydrate to the anhydrous tin (iv) chloride. Tin (iv) chloride is a liquid
compound prepared from direct combination of the elements. It is extremely hygroscopic and, from personal experience, not a very friendly compound. On
a longshot, if you had some magic reagent that reacted irreversibly with the water without any reaction with the tin, you might be able to do
something. Not likely I think.
AvB
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by AvBaeyer  | Sulaiman is quite correct. There is no practical way to convert the pentahydrate to the anhydrous tin (iv) chloride. Tin (iv) chloride is a liquid
compound prepared from direct combination of the elements. It is extremely hygroscopic and, from personal experience, not a very friendly compound. On
a longshot, if you had some magic reagent that reacted irreversibly with the water without any reaction with the tin, you might be able to do
something. Not likely I think.
AvB |
Thionyl chloride *might* work.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by AvBaeyer  | Sulaiman is quite correct. There is no practical way to convert the pentahydrate to the anhydrous tin (iv) chloride. Tin (iv) chloride is a liquid
compound prepared from direct combination of the elements. It is extremely hygroscopic and, from personal experience, not a very friendly compound. On
a longshot, if you had some magic reagent that reacted irreversibly with the water without any reaction with the tin, you might be able to do
something. Not likely I think.
AvB |
Thionyl chloride *might* work. |
But even if it did, that would be a waste of thionyl chloride. Setting up a
chlorine generator and passing the gas over tin metal shot is much easier. I've done it before, and contrary to the Wikipedia page, it doesn't even
require an external heating source. Just make sure your glassware is dried before you start and you use a drying tube between the chlorine generator
and the reaction vessel, or you'll get a mixture of anhydrous and hydrate.
AvB is right though- it isn't a very friendly compound. In air it produces heavy, white tin(IV) oxide / HCl smoke, which is quite irritating. You'll
want to transfer it to a nice dry vial or ampoule and seal it up as fast as possible.
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
https://geocitieschloratesite.000webhostapp.com/chlorate/sta...
Some stuff on stannic chloride. It does not answer your question though.
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
??????????
KClO8?!?!
What the hell is that??
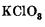
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I would assume it's a bad scan of KClO3.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Act like you are dehydrating MgCl2.6H2O (see http://web.mit.edu/dsadoway/www/106.pdf ).
Try heating under a blanket of hydrogen chloride at the proper partial pressure of HCl (to stop the normally preferred hydrolysis reaction).
Not likely easy, however.
|
|
|