CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
weird reactions
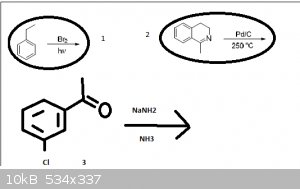
Hi guys I have 3 problematic reactions can you help me? please 
in the frist one: radical substitution - Br will substitute α (on ethyl and closer to benzene) hydrogen or other??
2: I dont have any idea what can happen   
3: will there work addition-elimination mechanism (even when i have I- and not M-???
|
|
|
Dr.Bob
International Hazard
    
Posts: 2821
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Why don't you propose some ideas and then we can review them. Or explain what the pros and cons are of each possible reaction or product. Most
people here don't want to do the work for other people without them trying first.
ie, in 1) the alpha proton may substitute more due to X and less due to Y, but the beta position will be more reactive due to z.
|
|
|
Texium
|
Thread Moved
25-1-2018 at 06:22 |
CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
ok thanks, 
I think that in 2nd case palladium is good for attracting hydrogens and next to nitrogen (in cycle) is saturated C-C bond so it might rip off those
hydrogens and make more stable aromatic heterocycle.....?
and in third case i think it should work because on meta carbon should be parcial positive carge, am I wrong?
CxD
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by CrossxD  | ok thanks, 
I think that in 2nd case palladium is good for attracting hydrogens and next to nitrogen (in cycle) is saturated C-C bond so it might rip off those
hydrogens and make more stable aromatic heterocycle.....?
and in third case i think it should work because on meta carbon should be parcial positive carge, am I wrong?
CxD
|
Pd/C is used as a catalyst for reduction using H2, try have a look at all of the atoms around the molecule and see which ones can be reduced. Also
consider that aromatic systems cannot be reduced via this method at low pressure (is there any indication as to whether it is high or low?), and that
a carbon atom saturated with hydrogen cannot be reduced further.
For number 3, I’ll give you two hints. Nucleophilic aromatic substitution, and Schiff base. Technically three, if I tell you that these two occur
separately.
[Edited on 25-1-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
I know that saturated carbon cannot be reduced but i dont have H2 in that reaction.... so I thought that palladium would dehydrogenate at high
temperature and make double bond and h2 and that bond would participate in cojugated double bond system to stabilize it
number 3... I get it  i forgot about Schiff there will be imine right? i forgot about Schiff there will be imine right?
CxD
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
The 3rd example is perhaps proceeding through a benzyne derivative. Noting that the acetyl group is in the metaposition it does not allow for a
resonance stabilized meisenheimer complex (a.k.a. Sigma complex), although it could proceed through one, but it simply wouldn't be stabilized by
resonance.
http://www.ochempal.org/index.php/alphabetical/a-b/benzyne-m...
http://www.ochempal.org/index.php/alphabetical/s-t/snar-mech...
[Edited on 25-1-2018 by Sigmatropic]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Pd/C implies hydrogenating conditions. I guess that's one of those things you learn with experience. There just isn't much use for Pd/C without
hydrogen present.
For the first one, the conditions clearly indicate this will be a free radical reaction. However, what usually happens is bromine will pull off a
hydrogen to form HBr, then the other bromine will occupy its place. I guess I'm not sure if that counts as a substitution or not.
[Edited on 1/26/18 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | Pd/C implies hydrogenating conditions. I guess that's one of those things you learn with experience. There just isn't much use for Pd/C without
hydrogen present.
|
Incorrect. Note that in the reaction drawn above the temperature is extremely high and no hydrogen is given as a reagent. This could not possibly be a
hydrogenation reaction.
Instead, at high temperatures palladium on carbon functions as a dehydrogenating reagent. It converts cyclohexenones to phenols, and (usefully in this
case) dihydropyridines to pyridines. Thus, the product of the corresponding reaction is the corresponding isoquinoline plus a mole of hydrogen.
[Edited on 26-1-2018 by Cryolite.]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I have literally never seen a single reaction in literature that was done with Pd/C without hydrogen.
But I guess there's a first time for everything.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|