VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Addition of HCl to Propenoic acid
I am on chapter 6 of my orgo book and unfortunately the AP chem teacher at my high school recently quite his job. I already asked a uni student who is
taking some chem courses and I have yet to receive an expiation for this reaction. I also googled the problem and came up with no answers or
explanations. Help me Science Madness you are my only hope.

Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
I found this on the Wikipedia article for 'nucleophilic conjugate addition'. I know it's not the same mechanism as what is occurring here, but it
would explain why only the 3-chloro isomer is formed. Hopefully more insight and/or improvements will come this way, it's peaked my interest now.
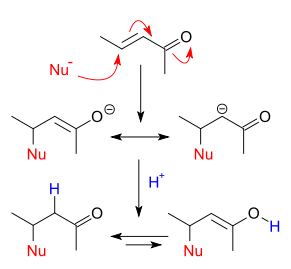
[Edited on 9-12-2017 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
I would assume LearnedAmateur's mechanism is correct, as only HBr can undergo free-radical addition, ending in a product opposing Markovnikov's rule.
Another one of the many exceptions in organic chemistry  . .
The philosophy of one century is the common sense of the next.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LearnedAmateur  | I know it's not the same mechanism as what is occurring here, but it would explain why only the 3-chloro isomer is formed.
[Edited on 9-12-2017 by LearnedAmateur] |
What makes you say that?
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Thank you. I worked out mechanism on paper and it seems to work.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Propenoic acid contains an a,b unsaturated carbonyl, which is what is required for this particular reaction. I know that the hydroxyl group generates
a resonance structure which could mean a completely different mechanism, but this is the closest thing I can find.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Since chloride isn't that great of a nucleophile, I wouldn't be surprised if the first step was the protonation of the carboxylic acid group, which
would give a cation that would resonate and put some positive charge on the beta carbon.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Could anyone help me with this problem as well? Thanks.
[Edited on 10-12-2017 by VSEPR_VOID]

Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|