mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Synthesis of diisobutyl 3,9 perylenedicarboxylate
Im interested in making compound named diisobutyl 3,9 perylenedicarboxylate. And i have an idea but i dont know will it work. I want to make dicarboxy
perylene by scholl reaction with 1 naphthoic acid and lewis acid but i dont know how to make naphthoic acid. Maybe better way is to make dicarboxy
perylene from perylene itself. Then i want to esterificate it with isobuthanol.
There is picture of this compounds structure. It seems easy to make.
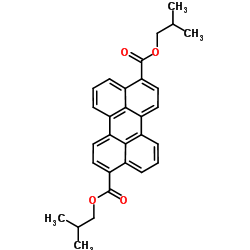
[Edited on 5-11-2017 by mackolol]
[Edited on 6-11-2017 by mackolol]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
All i want to know is if it work and how can i make 1-naphthoic acid. Can somebody help me?
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
It looks like it might be a colorful compound - but why do you want to make it?
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Green chemiluminescent dye and i think it could be easiest green dye to synthesise. Other two i know is coumarin 7 there is topic about it and 9,10bis
phenylethynyl anthracene
|
|
|
Boffis
International Hazard
    
Posts: 1901
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Mackolol, I have done a bit of research and it looks like there are two routes to the basic acid. The first is from 3,9-dibromo or dichloroperylene,
halogen displacement to the dinitrile using cuprous cyanide (easily prepared from ferrocyanide see SM thread) and then hydrolysis of the cyano group
to and acid. Or alternatively from perylene via a double Freidel-Craft acylation with acetyl chloride to 3,9-diacetylperylene and then alkaline
oxidation to the required diacid.
The problem in the first case is the source of 3,9-dihaloperylenes, however, although I haven't found a definite procedure yet it appears that direct
bromination in a fashion similar to the procedures for naphthalene (eg in Vogel) yields mainly the 3,9 disubstituted product. I have found a reference
to the preparation of perylene from bis-(2-naphthol) which is prepapred by oxidising 2-naphthol with ferric chloride etc. The yield is low however but
there may be better methods (maybe ask nicely of someone on the references thread and they will run a reaxys search for you  ). ).
Here are a few refs about perylene to keep you busy:
Scholl et al.; Berichte C.; 1910, v43, p2203
Hansging & Zinke; Monatsh.. ; 1919; v40 p404
Zinke et al; Monatsh.. ; 1932; v61 p1
Clar; Berchte C.; 1932; v65, p846
Morgan & Mitchell; JCS; 1934; p534. (this last refs contains a reference to a simple prep for perylene but the yield is low)
For Perylene-3,9-dicarboxylic acid check out:
Pongratz; Monatsch, 1927; v 48 p585 & 1929: v52, p9
Pongratz et al.; 1933; v62 p71
Another possible route is via the degradation and reduction of perylenequinone vat dyes or perylene 3,4,9,10 tetracarboxylic acid which may partly
decarboxylate at high termperatures. There are lots of patents on these two groups, most of the ones I have found so far are in German. Can you read
German?
For the preparation of the tetracarboxylic acid try:
German Patents: 394794; 408513 and 412122
I have a book about the synthesis of VAT dyes and there may also be something in there. The significance of a route via perylene vat dyes is that
inorder to be commercially useful it had to be easy to prepare in 1900s via a simple mix-n-bake type procedure.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
1.Napthalene to perylene using scholl reaction -http://onlinelibrary.wiley.com/doi/10.1002/cber.191004302175...
2.perylene to 3,9-dibromoperylene - http://onlinelibrary.wiley.com/doi/10.1002/jlac.199719970218...
3.Convert DBP to a double grignard reagent and react with dry ice to get dicarboxylic acid
4.esterification with isobutanol -http://www.tandfonline.com/doi/full/10.1080/00397911.2013.83...
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Thank you and how would it look like if i will use acetyl chloride and if potassium permanganate is enough to ozidise it to acid?
|
|
|
Boffis
International Hazard
    
Posts: 1901
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
If you are going to go via FC acylation then acetyl chloride and AlCl3 looks good. The usual method of oxidation of acetophenone to benzoic acid is
with sodium hypochlorite and this reaction ought to be analogous though I think KOH/NaOH and KMnO4 would work too but is more expensive.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
1-napthoic acid might not undergo the scholl reaction since COOH is a deactivating group
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Do you have any other source than onlinelibrary? Because i dont have access to this and dont wont to pay.
|
|
|
plastics
Hazard to Others
  
Posts: 141
Registered: 6-11-2009
Member Is Offline
Mood: No Mood
|
|
Attachment: scholl1910.pdf (355kB)
This file has been downloaded 510 times
Attachment: schlichting1997.pdf (1.4MB)
This file has been downloaded 511 times
|
|
|