kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Nitration of phthalic acid
Acronyms:PHA - phthalic acid;PHAN - phthalic acid anhydride; 3NPA 3-nitrophthalic acid; 4NPA 4-nitrophthalic acid
Almost all methods of preparation of 3NPA (and 4NPA) are based on nitration of PHAN.
But sometimes PHA may be more available for someone, than PHAN is. Available and cheap PHA esters or salts can be converted easily to PHA. Besides,
PHA is easier to obtain in pure and stable form (simple purification from hot/cold water). Unfortunately, PHA is far less reactive under standard
nitration process and even small amount of water present in reacion mixture is harmful. Then large excess of HNO3 (concentrated, >95%) is needed,
with or without addition of H2SO4. When too much water is present, nitration process becomes very slow and oxidizing properties of such mixture
increases - it generates NOx fumes and water. This problem can be bypassed by known trick: using powerful nitrating KNO3-H2SO4 mixture.It generates
HNO3 (its solution) in situ, without production of water.The trick is known for ages, but somehow I have seen only one example of using it (some
Russian forum) for nitration of PHA. The procedure described below was performed more than 20 times, without any problems and runaways. However,
special care should be taken because of potentially dangerous (violent reactions with organics) properities of hot KNO3-H2SO4 mixture. The procedure
was performed with 8-12g samples of PHA, but seems scalable. At any stage of reaction, it can be stopped and continued the next day (for example). The
reaction gives similar output of 3NPA as from nitration of PHAN, about 25% molar (counting on PHA), but it strongly depends on the method of treatment
of the post-nitration mixture. The yield ~10 % is nothing unusual.
Part 1, nitration of PHA
23g of ~96%(or more conc.) H2SO4 was added to 8,0g of PHA in 100 ml RBF with ground glass joint .The acid quickly dissolves upon manual stirring with
generation of some heat, but temperature rises merely to ~30C. Resulting solution is clear, colorless, water-like but slightly oily. It is set aside
(cork stoppered), until it reaches room temperature and 6,5g of KNO3 (powdered) is added in ~1g portions(1). Strong manual stirring (not shaking)
after each portion is needed to avoid creation of one big salpeter lupm. The mixture becomes warm again, slightly more than earlier, but no cooling is
needed. The warm mixture is slightly fuming (HNO3) when the flask is not stoppered. When all KNO3 is added, the flask with more or less dense
suspension is palced in water bath at 60-70 C. The flask is simply glass cork stoppered, no reflux and mechanical stirring apparatus is needed(!).It
is stirred manually from time to time, several times the cork is lifted for a second to equalize the pressure. After 15-60 minutes (2)the mixture
becomes slightly yellowish water-transparent liquid. Heating at ~70 C is continued, after another ~1-2 hours this clear solution becomes turbid and
white sediment gradually formes. Soon before it, tiny bubbles of some gas are visible in the liquid, especially during stirring, but practically
without foaming. From now, the temperature can be increased to ~80 C and heating continued for ~3 hours, with (manual) stirring from time to time (+
lifting the cork). There are practically no NOx fumes during reaction (as should be expected in good nitration) and no hood is needed (!). In one
experiment, weight of RBF before and after this ~5 hours heating perion was compared - the difference was smaller than 0,1 g. Finally, the flask is
removed from water bath and set aside to cool down.
The cooled mixture is thick suspension of crystals, creamy-white coloured. When the mixture looks more liquid than solid, it means that the liquid is
supersaturated. Stirring with glass rod soon causes crystallization and the whole mixure attains a pasty consistency.The mixture contains 3NPA,4NPA,
H2SO4,KHSO4,H2O, most possibly (NO)HSO4 in small amount, nitrated phenols (picric acid) also in small amounts.
(1) when KNO3 is added quicky to still warm PHA solution, some precipitate appears as fine white suspension, the most possibly it is PHA; seems that
solvation precesses are not finished (the equilibrium is not established yet), but it has no effect on further course of nitration reaction.
(2) in two cases, used PHA was possibly contaminated by small amount of KCl (weak odour of HCl when H2SO4 is added) and I have impression that in
these cases the reaction runs quicker, but I am not sure of it. However, the results were the same as in the other runs, with pure PHA.
--------------
Unfortunately, liberation of 3NPA from this nitro-mixture is problematic... I did many modifications in this direction, but I could not find optimal
solution of this problem. More about it in Part 2 (coming soon).
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice write-up kmno4; I haven't tried this with phthalic acid but I did some preliminary work on nitration of terephthalic acid a little while back
(when I posted all the stuff on terephthalic acid). I used similar quantities, 10g TPA in 50ml of 98% sulphuric acid and then added about 10g of dried
sodium nitrate (roughly 2 fold excess). This was heated on a stirrer hotplate for 2 hrs before drowning in ice and water. The nitration of
terephthalic acid has the advantage over phthalic acid that there is only on possible isomer but it appears very difficult to nitrate and initially I
struggled to find a way to test the product which looked exactly like the starting material and was equally infusible. My plan now is to use the same
technique that I used for the terephthalic acid and terepthalamide derivatives that I prepared, the back-titrated equivalence value, the fully
nitrated acid should require less alkali to neutralize it than the partially nitrated acid per unit weight. I have yet to try this on my produc but I
fear that it is mainly recovered TPA for various other reasons. 
I also tried this method to dinitrate 2-chlorophenol. The target was 2,4-dinitro-6-chlorophenol and then by partial reduction
4-nitro-6-chloro-2aminophenol. Th nitric acid route works well with standard conc nitric acid (70%) and sulphuric acid and is high yielding but nitric
acid is getting difficult to source now so I tied sodium nitrate again but the results were poor and the product difficult to isolate, it appears to
be much more soluble in the sodium sulphate -dilute acid mixture than in dilute sulphuric acid alone.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Part 2, 3NPA
Cooled (r.t.),pasty post-nitration mixture is transfered (several portions) with aid of glass (polypropylene/ethylene...etc) rod, into beaker
containing 35 g (1) of ice-cold water, with stirring from time to time (with another rod).
There is some heat generation (no need to cool it down), the water becomes warm, some nitrogen oxide (NO) is liberated (possibly hydrolysis of
(NO)HSO4), the solution becomes intensively yellow-green coloured (2), some precipitate separates.
When almost whole mixture is transfered out, several portions of few cm3 coloured solution is added to RBF and the rest of pasy mixture is washed out
and added to the beaker.
Coloured solution, with lumps, is strongly stirred to dissolve all lumps, there should be clear solution and powdery sediment on the bottom.
Next,it is placed in some cold place ( ~15 C max) for at least 24 hours(3). During this time, more sediment separates; when precipitation is finished,
the mixture is filtered (sintered glass, vacuum). The filtrate is rejected (4), the precipitate is dried at 80-100 C to constant weight: about 15 g of
white powder (with slight green shade) should be obtained (5).
Up to this moment, everything is simple and clear, problem rises when 3NPA is going to be extracted from this powder.
Fractional crystallization from water seems to be the simplest solution, based on solubilities of nitroacids.
Unfortunately: mixture of 3NPA and 4NPA in water has very "bad" properities.
Solubility of 3NPA in water at 10 C is 0,9 g/100g, but in the presence of even small amounts of 4NPA, solubility of 3NPA rises
and solid phase, in equlibrium with solution, above relatively low concentrations is not 3NPA but adduct of 3NPA, 4NPA and water.
So, crystallization from high concentrations (smaller volume of solution) gives mixture of 3NPA and 4NPA, crystallizations from low concentrations
(larger volumes of solution) gives low amount, but pure, of 3NPA (more info in attachment).
It seems that there is no good way to separate 3NPA/4NPA by treatment with water solutions....
All organics can be extracted from solution of 3NPA/4NPA by THF (and other ethers), but there is no clear cutoff between extraction 3NPA and 4NPA,
possibly because of complex (adducts) formation.
There are patents describing separation of these acids using dioxane (3NPA gives crystalline complex with dioxane and can be separated from solution
of 4NPA in dioxane, see for example CN101134728B).Even if it really works, it makes whole procedure more complicated and time consuming.
Solution of crude mixture in water, partly saturated with NaCl gives sligtly better amounts of 3NPA, but I am not satisfied with it, part of 3NPA
still stays in solution.
Maybe I do something wrongly, or not in the way as I should do - that is why I give only general directions, without whole experimentals.
To obtain few grams of relatively pure 3NPA, prepared earlier 15 g sample is dissolved in 100 g of hot water, with about 20 g of NaCl.
Clear green solution is cooled and set aside for at least 3 days (at ~10 C). Crystallization of 3NPA is very slow, even after 1 day may be no
crystals. Prepared 3NPA gives correct m.p. but if not, it should be crystallized from small (~10cm3) amout of hot water. Solutions of 3NPA are almost
coloureless, green coloration is caused by 4NPA.
------
Any ideas about separation of 3NPA and 4NPA are welcome.
(1) when more or less water is used, more or less pracipitate is obtained (eg. 21 g or 9 g); however no improvement in 3NPA yield is observed when
more precipitate is prapared
(2) the "yellow part" of this colour is caused (most possibly) by picric acid
(3) unfortunately, equilibrium sets up very slowly in case of mixture 3NPA and 4NPA with water, I recommend 48 h hours of standing
(4) besides inorganics, it contais 4NPA and unknown amout of 3NPA (and by-products): any attempt to recover 3NPA from it failed (lack of skills ?)
(5) this amount strongy depends on exact conditions (amount of substrates, water, time, temperature), see (1); this is mixture of 4NPA, 3NPA, KHSO4,
H2SO4,H2O
ps. Boffis, I do not have TPA at hand, I will try to prepare some from PET hydrolysis and test its nitration, just for fun.
Attachment: 3np_4np_H2O_ system.pdf (269kB)
This file has been downloaded 602 times
Слава Україні !
Героям слава !
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
I need 100-200 g of 3-nitrophthalic acid for my non-luminol scientific project, so I followed kmno4's procedure.
My yield was 18 %, m.p.=218-220 C.
There were 2 differences: I washed the precipitate just after the nitation and the target product with water (0-4 C). May be, I shouldn't have and
these washings led to such a low yield.
So, kmno4's method really works, but I find this classical nitration unsatisfactory because of 2 reasons.
1. The yield is too small.
2. H2SO4 is a scheduled substance in a country I live in, so its usage in this synthesis is just a waste of the limited reagent. Thinking about
СH3SO3H :-D.
I tried a literature search. These are the results:
1. One another classical method of 3-nitrophthalic acid preparation is via naphthalene nitration, that can be done with HNO3. Too long for me.
2. There's a nonclassical Bi(NO3)3-SiO2/THF/MW-nitration of phthalic anhydride with 75-85 % yield (see attachment). All regents are avaliable for me,
but microwave reaction seems to be difficult, since I have no microwave owen. And even if I have, I cannot do this work at home.
3. MOOSAVI SEYED MOJTABA and SHAHSAVAN EBRAHIM discribed phthalic acid nitration (40 % yield) done by 65 % HNO3 (https://www.sid.ir/FileServer/SE/240E201417175.pdf), but with no details. I wrote them, but no one answerd.
4. Some information about 3- and 4-nitrophthalic acid solubilities see in attachment.
5. kmno4 wrote about H2SO4/MNO3 nitration discribed in russian forums. See attachment (taken from here: http://chemistry-chemists.com/forum/viewtopic.php?f=4&t=...), sorry for no translation.
Attachment: Bi(N03)3-SiO2-THF-MW nitration.pdf (96kB)
This file has been downloaded 503 times
Attachment: solubilities.pdf (44kB)
This file has been downloaded 471 times  
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
What else is there to do with 3-NO2-PHA? What country are you in? I made some of that about 40 years ago towards luminol, but can't remember the
details. I did find the flask of luminol recently, however. That was one of my first bits of mad science. I do have some PHA and derives, if
anyone in the US needs any...
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The problem is not the yield of nitro acid but the ratio of 3 to 4 nitro acids and their separation. The are numerous proposed method but all have
their problems and complexities. The crystallisation of the mixed acid from a concentrated solution gives a mixed adduct of 3 and 4 isomers plus water
so little is gained by this procedure. If a more dilute solution is used a small amount of fairly pure 3 isomer will crystallise out but when you
concentrate the residual solution for another crop you get a mixture. In Vogel he descibes the separation via the esters, one isomer forms a neutral
water insoluble ester while the other gives only a half ester which is soluble in cold dilute alkalis.
I have ofter thought about trying separation by fractional crystallisation but given that this is such an obvious method and I have never seen any
references to such a process it may not work either.
Attached is a patent for the separation of 3 and 4-nitrophthalic acid using an organic solvent and partial neutralisation of the mixed acids.
[Edited on 5-12-2019 by Boffis]
Attachment: US4284797.pdf (562kB)
This file has been downloaded 458 times
|
|
|
Fery
International Hazard
    
Posts: 1016
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Better yield when started from phtalanhydride and fuming nitric acid, but I did not try it myself yet.
http://www.orgsyn.org/Content/pdfs/procedures/CV1P0408.pdf
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Fery,
Even starting with the anhydride the overal yield of pure 3-nitrophthalic acid is only about 30-32% because, as kmno4 has pointed out above,
separating the residue is very difficult due to association adducts. Mostof the people on the forum have already seen the OrgSynth articles and some
the original articles by Bogert & Boroschek, Langmuir, Marquius etc. The first of these authors discuss in great detail the merits of the
different techniques. The eventually settled for the 30% yield of 3-nitrophthalic acid and then prepared the 4-nitro isomer by oxidizing
4-nitrophthalide which presumably, though not stated, was prepared by nitration of phthalide which I believe is prepared by the reduction of phthalic
anhydride. So a very complex process indeed.
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | The problem is not the yield of nitro acid but the ratio of 3 to 4 nitro acids and their separation.
[Edited on 5-12-2019 by Boffis] |
For me the yield is the major problem. As u can see, I obtained rather pure 3-NPA, but only about 2 g from 8 g of PA!
Quote: Originally posted by Boffis  |
Attached is a patent for the separation of 3 and 4-nitrophthalic acid using an organic solvent and partial neutralisation of the mixed acids.
[Edited on 5-12-2019 by Boffis] |
Thanks. Acetone and methylethylketone are also scheduled, but I can try i-PrOH.
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dr.Bob  | | What else is there to do with 3-NO2-PHA? What country are you in? I made some of that about 40 years ago towards luminol, but can't remember the
details. I did find the flask of luminol recently, however. That was one of my first bits of mad science. I do have some PHA and derives, if
anyone in the US needs any... |
I'm going to use it as a synthetic intermediate, nothing special. And I'm far away from the USA  . .
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | @Fery,
and then prepared the 4-nitro isomer by oxidizing 4-nitrophthalide which presumably, though not stated, was prepared by nitration of phthalide which I
believe is prepared by the reduction of phthalic anhydride. So a very complex process indeed. |
Phtalimide is nitrated predominately in position 4, so this route is rather simple:
phthalic acid (thermal dehydratation)-> phthalic anhydride (urea)-> phtalimide (KNO3/H2SO4) -> 4-nitrophtalimide
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Brij-09 phthalide NOT phthalamide.
This is phthalide:
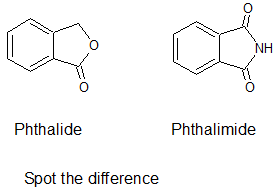
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
I know what is phthalide and what is phthalimide. I have recently synthesized both of them. )). Once again: phthalimide is predominatly nitrated in
position 4. So if u need 4-NPA, u don't have to make phthalic anhydride -> phthalide reduction. Just make phthalimide and nitrate it.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
If basic chemicals, like H2SO4, are unavailable for you, the most economical way is just buying it. It is not very expesive, especiallly from chinese
suppliers.
ps. when I am thinking about preparation of this acid, I feel a paind everywhere, not only in the ass.
Currently I am making a sample of malonic acis for my fellow (clock reaction) and its final extraction is a sitter, comparing to 3-NPA.
Слава Україні !
Героям слава !
|
|
|
Brij-09
Harmless

Posts: 12
Registered: 30-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  |
If basic chemicals, like H2SO4, are unavailable for you, the most economical way is just buying it. It is not very expesive, especiallly from chinese
suppliers. |
I didn't say it was unavailable. ))
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This topis was named "Nitration of phthalic acid", not "Problems with watched and/or expesive chemicals" or "The best method of obtaining 3-NPA.
Presented method is very, very cheap, even on 10% yield level, 18% is not bad at all. Of course, enlarging scale of production creates additional
problems, negligible at 10g (let's say) scale.
I think you create some artifical problems, that is all. Good luck in obtaning your product, in this or that way...
Слава Україні !
Героям слава !
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | ... a patent for the separation of 3 and 4-nitrophthalic acid using an organic solvent and partial neutralisation of the mixed acids.
|
Yes, I knew it earlier, but I had no appropriate pH meter to try this.
Fortuitously, I have found recently there are available very cheap, digital pH meters with glass "electrode" at ebay ( and similar sites).
I have bought something like this (foto from ebay):

I was surprised, because this device gives stable and reproducible readings (thank you, PRC !). For interested, the plastic casing is made from
polystyrene (or ABS) material.
Experiments are in progress, I am going to report the effects soon.
Слава Україні !
Героям слава !
|
|
|
Fery
International Hazard
    
Posts: 1016
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Boffis, thx for posting the US4284797.pdf
The separation method seems to use the advantage of different pKa1. Then solubility of free acids in organic solvent while solubility of monosodium
salt in water phase.
I found these values, both are in water at 25 C:
3-nitrophthalic acid pKa1=1,88 (https://www.chemicalbook.com/ProductList_en.aspx?kwd=3-Nitro...)
4-nitrophthalic acid pKa1=2,11 (https://www.chemicalbook.com/ChemicalProductProperty_EN_CB34...)
the pKa difference seems not to be too much, though the method in the patent claimed quite efficient separation of the 2 isomers
they used methylethylketone, fortunately also more solvents are mentioned there, widespread acetone, ethylacetate, ethylene glycol, aliphatic alcohols
C1-C5, etc (preferred solvents MEK, ethyl acetate)
NaOH / NaHCO3 was used to extract 3-isomer first and finally remaining 4-isomer in a form of monosodium salts from the organic phase, salts separated
out in water phase (that's why the solvent has to be miscible with at least a little of water, some amount of water was initially added to the solvent
and that water separated out with the monosodium salt of the acids)
When dissolving the mixture of both 3- and 4- isomers in organic solvent (MEK) the pH was close to 0 (how did they measure it if the water phase was
not separated yet??? - just my thought), then pH was slowly raised by partial addition of NaOH and later NaHCO3 until pH 2,8 during which the 3-isomer
separated as monosodium salt insoluble in organic phase but soluble in water phase
addition of NaOH initially had an advantage of not releasing CO2, but closer the critical pH 2,8 it was better to add NaHCO3 which had a disadvantage
of CO2 frothing
in pH range 2,8-3,4 they separated mixture of both isomers (to be reused in next batch separation) and above pH 3,4 they separated 4-isomer by
addition of NaHCO3 until pH raised to 4,5 (I believe in home lab it is enough to observe CO2 evolution at this moment)
strange that they claim somewhat different pH range, maybe the pKa1 of the 4-isomer is not 2,11 as in the link I posted but more??? somewhat like 3,4
???
also for ethylacetate, unlike MEK I wouldn't use NaOH, just NaHCO3 to prevent hydrolysis - wouldn't be NaHCO3 preferred for MEK too to prevent aldol
condensation (perhaps in cold environment the condensation is slow enough???)
KMnO4 - looking forward your results of your experiment! The authors of the patent used close to 1:1 mixture of both isomers, so maybe even without pH
measuring if adding less than 50% of the base (NaHCO3) quite pure 3-isomer should be obtained? Most of people here are interested predominately in the
3-isomer and do not have too much usage for the 4-isomer.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
... I am still alive, the experiments are finally finished.
From the perspective of time, I must admit that some part of them was just a waste of time, but even these failed experiments were quite informative

The results are in development, but now I only say that two solvens were tested: ethanol and isopropanol. The rest of recommended solvents are not
resistant to reaction conditions (pH), or too expesive, or may damage (swelling) the casing of PH-meter.
The key factor is, besides controling pH, keeping water content at rather low concentration. In case of ethanolic solutions, increasing water content
up to ~15% (value from density measurements) gives clear homogeous solution, without precipitates, at pH ~3.
It took a lot of time to me to realize how harmful is careless increasing water content  However, results of precipitations in ethanol and isopropanol are very similar. However, results of precipitations in ethanol and isopropanol are very similar.
Because isopropanol is much cheaper than ethanol (especially anhydrous), the rest of my experiments were performed in this medium. More details will
be given in few days.
Слава Україні !
Героям слава !
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Does the pH meter work in anhydrous conditions? If so what readings do you get? Do you have some standards to check?
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Does the pH meter work in anhydrous conditions? |
I do not know. The pH meter I have is provided for water solutions, possibly it would give some readings in anhydrous conditions..... The theory of
glass electrode is rather complicated and I forgot almost whole of it 
However, in solutions containing even less than 1% of water, it behaves "as if" in pure water: readings are stable and set-in quickly (seconds). The
pH meter presented above is not thermally compensated, but one can correct reading by small screwdriver (included). Two buffer powders are also
included in the set, but I used tap water as standard - it was checked, many times, that it has pH=7,0 everyday 
The main drawback (beside low thermal stability of readings) of this pH meter is plastic case. I am sure it is not resistant to ketones, esters and
aromatic hydrocarbons, as it is made from polystyrene-like material. But for water and alcoholic solutions it is OK.
Слава Україні !
Героям слава !
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
During the experiments, new improved proportions for nitration of PHA was developed.
Namely:
5 g of PHA + 12,5 g of conc. H2SO4 + 3,42 g of KNO3
Instead of KNO3, NaNO3 can be used (2,88 g). But KNO3 has better solubility properties, because under reaction
conditions, only highly soluble KHSO4 is formed, contrary to NaNO3 which gives less soluble "double salt" NaHSO4xH2SO4. However, final results are the
same: the same amount of 3NPA is obtained. The semi-solid mass obtained after nitration was mixed with 18,3 g of H2O. The solid obtained (see the post
at the beginning of the tread) weighs about 11 g. The yellow filtrate contains 30-40 % of formed 4NPA, but almost no 3NPA - no improved amount of 3NPA
is obtained after working-up the filtrate. Prepared 10-12 g sediment is congruent for further work-up. 95% isopropanol (IPA) was used for all further
preparations. More concentrated, it gives bad looking, muddy, and not well resolved sediments. Much less content of IPA may give too low amounts of
the sediments. For this 11 g of post-nitration solid, 100 cm3 of the IPA solution was used. This volume seems to be appropriate for given amount of
the solid, but it is not very critical parameter. An attempt was made to reduce amount of introduced water, by neutralizing the wet solid with Na2CO3
and drying.
Unfortunately, some semi-solid eutectic mixture of salts is formed. This yellow mass is very sticky and adhers to everything, playing with it is
loosing the time. So, the solid is placed in some twist-off jar, IPA solution is added and mixing on mag.stirrer is started. Without delay, about
1,5-2 g of solid NaOH is added in form of powder or granoules, not pellets.
It is because, in this low-water content mixture, dissolution rate is low. NaHCO3/Na2CO3 is not recommended - its dissolution and reaction is much
slower than in NaOH case, CO2 formation causes additional problems. When all NaOH is dissolved (it is important), pH of obtained solution is measured
: the goal is to obtain value 0,8-1. When it is too low, small amout of NaOH is added... etc., when it is too high, few droplets of conc. H2SO4 are
added. The result is yellowish-green liquid with dense, white sediment on the bottom. It is filtered off, washed with few cm3 of 98% (or better) IPA
and dried (optionally). It should be completely white, yellow or orange points mark not dissolved NaOH. About ~5 g of inorganic salts mixture is
obtained and it contains nothing interesting --> lavatory.
More NaOH is added, in several portions, checking pH in the meantime (stirrig, stirring !!). The next stop is pH=2,7-2,8. The sediment formed is
filtered, washed and dried. About 3-4 g of white, with green shade, 3NPA salt is obtained. If it has markedly yellow color, it means that something
went wrong and it contains more than expected admixture of 4NPA salt. In this case, it should be returned to the mother solution, stirred and pH is
rechecked. More than 10 preparations was performed in this way and such yellow coloration is in 100% indication of NaOH overdose.
Prepared salt is dissolved in 10 g of hot water and 1,5-2 cm3 of conc. HCl is added. On cooling, some crystals of 3NPA may form, but it does not
always happen.Obtained transparent, yellowish-green solution is cooled to ~5 C and kept in this temp. for several hours. After this time, white
sediment should be present - 3NPA separates as crystalline dust. The liquid should be mixed several times with glass rod, to finish crystallization.
It may take additional hours. Finally, it is filtered. Obtained crude 3NPA weighs ~1,8-2 g. This amount is achievable when everything was done
correctly, if not, this yield may be in 1-1,5 g range. The filtrate was kept !
To obtain slightly better yields, recovery of remaining 3NPA was performed.
To the remaining IPA filtrate, NaOH is added again, but more carefully, to obtain pH=3,5-3,6. It is then cooled below 0 C (freezer) and quickly
filtered. Filtration can be slower than earlier, even if amount of sediment is lower, but still it goes fast.
About 1-2 g of intermediate fraction, but still 3NPA rich, was obtained. It is deeper colored than previous fraction.
When further precipitations are performed with small amounts of NaOH, very small amounts of solids are obtained, becoming more and more
yellowish-brown.
Obtained solid was added to the aqueous filtrate from 3NPA precipitation, some Na2CO3 added and evaporated to dryness.
The IPA filtrate, containing maily 4NPA, was partly distilled off, to obtain ~50 cm3 distillate. Prepared earlier solid mixture was mixed with this
distillate and NaOH treated, this time without isolation of inorganics (it saves a little time), to reach pH=2,8, cool below 0 C. Precipitate was
filtered... etc. It weighs ~2 g, but after dissolving in water and adding HCl, merely ~0,2 g of 3NPA is obtained.
Total weight of prepared (crude) 3NPA is then ~2,2 g : I was not able to obtain more than this, even after many modifications of given procedures. It
seems that no more than 0,1-0,2 g of 3NPA is lost during all operations.
Recovery of 4NPA.
It is much hadred to perform, mainly because of very fine suspension, which is formed when pH of IPA solution is set
to ~4,5. The sediment is yellow, when suspended in mother liquid. It looks like at least 10 g and does not separate
(almost) from the liquid on standing. Filtering is very painful - it takes almost hours under vacuum, even when IPA starts boiling, the filtrate goes
off slowly drop by drop. Measurable amounts of IPA evaporates during such filtration, in the end there is yellowish-brown mud in the funnel.
The mud weights ~5 g, but after drying, dissapointing ~2 g of 4NPA sals is obtained. Its colour is strongly dependant on exact conditions of
precipitation and - possibly - impurities: from dirty yellowish, through "beige" (description from the patent) to reddish. However, the color of pure
sample of 4NPA prepared later,surprised me : white powder, very easily soluble in water, giving yellow-orange solutions. In moist air, the powder
attains canary-yellow coloration at thin surface layer. Re-drying removes this color.
Photo galery.

Another samples, with better separation of 3/4 NPA salts


A - inorganics
B - 3NPA salts
C - intermediate fraction pH=3,5
D - I do not remember, the most possibly it is fraction collected between pH 3,5-4,5
F - sample of 4NPA salts
F1-F4 - samples of 4NPA salts; F4 is residue from IPA evaporation and is not very pure
E1 - sample of rather pure 4NPA
E2 - another sample of 4NPA is slightly reddish
The colors differs markedly, depending on light source. The pictures are made under daylight.
3NPA is white powdery solid in all preparations, nothing interesting to show.
Remarks.
- in almost all cases, when amount of precipitated 3NPA was smaller (eg. 1,7 g), more 3NPA is obtained from intermediate fraction
- separation of inorganics at low pH, gives better results than collecting all solids at pH=2,8
- pH should be checked at least twice during ca. 30 minutes, before filering; it may not differ more than 0,1 unit.
- all operations are conducted at 20-30 C (exept coolig in indicated cases)
- all weights are given after at least 80 C drying (to constant weight)
- when all precipitations are finished, IPA was distilled from the solution. Remaining solid weighs less than 0,5 g and contains mostly impure,
sticky 4NPA salts. Larger water content in origanl solution causes markedly larger weight of this residue.
- the distilled off IPA was 89-90% (from its density)
- composition of obtained solid 3NPA/4NPA salts does not correspond to any acidic or neutral Na salts of these acids; the patent treats them as
monosodium salts, but amounts of separated acids are far too small for such composition
- amount of prepared 4NPA is slightly larger than 3NPA
Conclusions.
Unfortunately, separation of 3NPA and 4NPA is not so complete as the patent says. However, some progress is achieved comparing to water mixtures. The
whole separation procedure is very time-consuming and requiers careful control.
In my opinion, it makes sense only in case of lager scale preparation. On the other hand, it is exellent exercise in such old-school separation
process. The difference between pKa1's of the acids in water is very small. It seems that such composition of the solvent, containing ~10% of water,
acts as differentiating solvent for these 3/4 nitroacids. Maybe ketonic cosovents act better and give better separation than alcohols, but it was not
tested. The only choise seems to be acetone, because of its price and availability, but I did not try this (expected condensation issues). However, I
think it is not very hard to perform, maybe in some, more or less near, future, just for fun... 
[Edited on 2-10-2021 by kmno4]
Слава Україні !
Героям слава !
|
|
|
|