fleroj
Harmless

Posts: 6
Registered: 30-7-2017
Member Is Offline
Mood: No Mood
|
|
"Recycling" polycarbonates for phenol (and derivatives).
https://www.ncbi.nlm.nih.gov/pubmed/16387239
Seems interesting:
| Quote: |
Some metal chlorides were shown to be catalytic active for the degradation of PC at 400 degrees C, which increased degradation conversion from 8.5% to
more than 58.3% |
| Quote: |
The main liquid product is phenol, p-isopropylphenol, diphenyl carbonate, and bisphenol A for all cases. |
[Edited on 1-8-2017 by fleroj]
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 122
Registered: 27-4-2021
Member Is Offline
|
|
Pyrolyisis polycarbonate give mixture phenol. Phenol and p-cresol is main products. But it produces also very acrid smoke due to benzoquinone
presence. Phenol difficult to separate from other phenol due to close boling point. Also it requires high temperature(700-800C) due to coke formation
in retort. This leads fast corrosion steel retort. About 100g phenol from 1 kg polycarbonate may produced
You can glycolysis polycarbonate in boling ethylene glycol to bisphenol a and distil it with acid cathalyst as zinc chloride. It produces mixture
phenol and p-isopropenylphenol which easy separate and give much larger yield phenol and not requires high temperature
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Just an FYI for those of us who are interested:
Polycarbonate can be gotten by soaking a cd in HCL overnight to dissolve the aluminium layer away.this gives a very undamaged crystal clear land of
pc.
Using hydroxide to destroy the aluminium also works but does something to the plastic leaving it unclear. I do like the idea of turning old CDs into
phenol.
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 122
Registered: 27-4-2021
Member Is Offline
|
|
About 15kg compact discs was pyrolyzed.4-5l tar obtained. Tar was distilled.Obtained about 1200 ml fraction b.p 180-194C and 500 ml fraction boiled
194-205C
Fraction 1 is mainly phenol(80%). It dark liquid as coca-cola color with phenolic odor.
Fraction 2 is mainly p-cresol with some ethylpheol. Properties as fration 1
Fraction 1 during storage changes color to dark red
if continue distillation p ethylphenol and other phenol may obtainned
As we can see phenol contaminated p-cresol if polycarbonate pyrolysed alone
Best problem solving glycolysis polycarbonate with boiling ethylene glycol with sodium carbonate adding to Bisphenol A. Bisphenol A may pyrolysed with
acid cathalyst as zinc chloride or phosphoric acid and tar consisting of phenol and p-isopropenylphenol can be distilled without problemm( difference
boiling point 40C)
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
so how does one go about removing Bisphenol A?
"BPA exhibits toxic, endocrine, mutagenic and carcinogenic effect in living organisms."
notoriously toxic, phenol is adorable in comparison
very cool with an OTC source of phenol, ive used it in low concentration dissolved in carrier oil as disinfectant with subliminal efficiency
what can you use phenol for making? its possible to make salicylic acid from phenol and supercritical CO2 with K2CO3 catalyst
|
|
|
Bmoore55
Hazard to Self
 
Posts: 85
Registered: 23-7-2018
Location: Texas
Member Is Offline
|
|
Polycarbonate Pyrolysis Fragments
Here is an example of the pyrolysis fragments of polycarbonates.
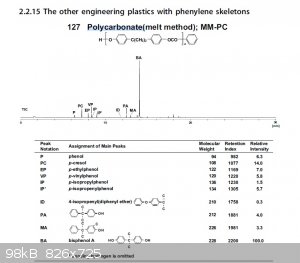
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 122
Registered: 27-4-2021
Member Is Offline
|
|
Distillation. Bisphenol A is high boiling point unlike phenol. Tar waste which contains bisphenol A can be burned in furnance
In extreme cases tar can be mixed with solvent and solution must be mixed with aqueous solution detergent and suspension merge into sewer
Bisphenol A have high LD 50(grams per kg weight) and low volatile. Phenol ld 50 about 100mg/kg
[Edited on 17-6-2021 by Hexabromobenzene]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Maybe a better solution would be for you to drink it so you'd stop giving stupid advice like this. Seriously, if you did this you deserve the worst
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 122
Registered: 27-4-2021
Member Is Offline
|
|
Throw chemical waste into the garbage tank can attract attention police. It very dangerous. Best solution burning waste if it possible or mix with
other household waste.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i can imagine if you head to the local dump with 10kg of bisphenol they will just automatically morph into hazmat suits same second you step out of
your car, phenol is quite toxic, but endocrine disruption is very scary stuff.
|
|
|
Hexabromobenzene
Hazard to Others
  
Posts: 122
Registered: 27-4-2021
Member Is Offline
|
|
Oh my God. Tons polycarbonate waste falls into the ocean, ground per year.It is slowly crushed and hydrolyzed into bisphenol a
Bisphenol a harmful on prolonged conact. It not bioaccumulative. Safe daly intake 0.25-1mg. Of course, it is not recommended to conduct experiments
with hydrolysis polycarbonate, pyrolysis bisphenol at home. If you follow accident prevention it safe.
Your professional impact not more than drinking water from office polycarbonate 20l bottle or tea from polycarbonate electric kettle. Some old plastic
kettle made from polyoxymethylene. How many formaldehyde drank people together with tea? Covers from some beer PETE keg still made polyoxymethylene.
With bear you drink some formaldehyde
Bisphenol A is xenoestrogen. Some transgender accept HRT. This is much more harmful than traces of BPA but it social norm now
Conclusion: If you are careful work with BPA safe. Main hazard is burns from distillation products BPA or polycabonate because it contains phenol
|
|
|